Abstract
Lithium-ion batteries (LIBs) represent the most promising choice for meeting the ever-growing demand of society for various electric applications, such as electric transportation, portable electronics, and grid storage. Nickel-rich layered oxides have largely replaced LiCoO2 in commercial batteries because of their low cost, high energy density, and good reliability. Traditional nickel-based oxide particles, usually called polycrystal materials, are composed of microsized primary particles. However, polycrystal particles tend to suffer from pulverization and severe side reactions along grain boundaries during cycling. These phenomena accelerate cell degradation. Single-crystal materials, which exhibit robust mechanical strength and a high surface area, have great potential to address the challenges that hinder their polycrystal counterparts. A comprehensive understanding of the growing body of research related to single-crystal materials is imperative to improve the performance of cathodes in LIBs. This review highlights origins, recent developments, challenges, and opportunities for single-crystal layered oxide cathodes. The synthesis science behind single-crystal materials and comparative studies between single-crystal and polycrystal materials are discussed in detail. Industrial techniques and facilities are also reviewed in combination with our group’s experiences in single-crystal research. Future development should focus on facile production with strong control of the particle size and distribution, structural defects, and impurities to fully reap the benefits of single-crystal materials.
Graphical abstract

Similar content being viewed by others
1 Introduction
In 1981, the Goodenough group first discovered that lithium cobalt oxides (LiCoO2) had high conductivity and maintained their structural stability throughout ≈ 0.5 Li+ (de)intercalation per formula unit [1]. Since then, after being introduced in the first commercial lithium-ion battery (LIB, energy density: ~ 150 Wh kg−1) by Sony, lithium cobalt oxides have been utilized in batteries as cathode materials. Lithium cobalt oxides continue to play a dominant role among many positive materials. LiNiO2, which has the same layered structure as LiCoO2, was first considered as an alternative cathode material given the high cost and scarce resources of elemental cobalt. Nevertheless, high-quality LiNiO2 samples are very difficult to synthesize and prone to chemical and structural instability [2,3,4], particularly at high cutoff voltages. Delithiated LiNiO2 easily releases oxygen even under normal operating conditions and undergoes exothermic reactions with organic electrolytes [5, 6]. Nickel-based oxides LiNi1–x–yCoxAlyO2 (NCA) and LiNi1–x–yCoxMnyO2 (NCM) are obtained by introducing cobalt and manganese (aluminum) into LiNiO2 to improve its structural and thermal stability [7,8,9]. However, although the high proportion of nickel in the family of nickel-based layered oxide materials can allow for increased gravimetric energy, the low reversible capacity of layered oxides with high nickel contents hinders their further application in commercial batteries. In particular, nickel-based cathodes (\(\geqslant\)80% nickel content) with high electrode densities that exceed ≈ 3.3 g cm−3 usually undergo structural collapse, exhibiting severe capacity fading as the gravimetric energy density increases [9, 12, 16]. Even so, next-generation batteries with nickel-rich layered oxide cathodes and graphite–silicon anodes are still expected to achieve cell-level specific energy densities approaching 350 Wh kg−1 by 2025 or earlier [10].
Many researchers have published excellent reviews detailing the research progress on understanding critical challenges that such cathode materials and corresponding strategies face to fulfill the goal of high energy density [11,12,13,14,15,16,17,18,19]. Most reviews have mainly focused on the failure of layered oxides caused by electrochemical cycling and storage along with the two conventional methods of coating and atomic doping. Other strategies, such as those based on core–shells, concentration gradients, and single-crystal structures, are only incidentally mentioned and considered undeveloped concepts. Conventional modifications temporarily postpone capacity fading and show reduced effectivity due to the migration of doped atoms or damage to the protected layer. The redesign of the morphological structure provides a promising strategy to radically counteract the inherent degradation associated with lattice distortion during cycling while exhibiting high potential for easy scaled-up production. Notably, single-crystal nickel-based layered oxides are believed to be beneficial for the better electrochemical stability.
The operation of LIBs with a nickel-based layered oxide cathode and a graphite anode is illustrated in Fig. 1a. The extraction of lithium ions from the layered structure accompanies the oxidation of transition metal ions during charging. In this system, interactive reactions among the lithium, cathode/anode, and electrolyte result in complicated interfacial chemistry. In particular, compared to polycrystal materials, single-crystal materials have a wider cutoff voltage window and can endure longer cycling and higher temperature (Fig. 1b). Regarding polycrystal materials, stringent operating conditions increase the contact area between the electrolyte and electrode materials due to the electrochemical generation of microcracks (pulverization), resulting in additional side reactions and even increasing the charge transfer resistance. Single-crystal materials can maintain their morphological integrity in the absence of anisotropic forces even if operated under extreme conditions, thus reducing gas evolution during electrochemical cycling. The origin, synthesis, development, and modifications of single-crystal nickel-based cathodes have been well reviewed from this perspective. This review aims to inspire new ideas regarding single-crystal materials and pave a path toward LIBs with high energy density.
2 Nickel-Based Positive Electrode Materials
2.1 Chemical Structure
Similar to LiCoO2, nickel-rich layered oxides [LiTMO2, (TM = Ni, Co, Mn)] adopt the α-NaFeO2 structure with a layered hexagonal structure (space group: R3m) [2]. As shown in Fig. 2a, this overall structure is composed of consecutive alternating [TMO6] and [LiO6] octahedrals wherein the lithium, TM, and oxygen atoms reside at the 3a, 3b, and 6c sites of the crystal structure, respectively. Given that the radius of Ni2+ (0.069 nm) is negligibly different from that of Li+ (0.076 nm), a small amount of Ni2+ and Li+ usually exchange positions. This phenomenon, which is termed cation antisite disorder, impedes lithium-ion diffusion in the layered framework [20, 21]. Figure 2b presents the Rietveld refinement of layered oxides with different nickel contents. Oxides with various nickel contents present general upward and downward trends corresponding to lattice parameters a and c, respectively. Cation antisites increase lattice parameter a, as indicated by the presence of weak (003) diffraction peaks. In the lithium layer, Ni2+, which has a small radius, decreases the lattice distance and tends to be oxidized into Ni3+/4+ during charging, thus causing local lattice collapse. In contrast, lithium ions sliding into the TM layer increase the lattice distance and obstruct Li+ insertion/extraction due to their large radius. A series of Li1–xNi1–xO2 in the solid solution state constitute LiNiO2. The ideal layered structure LiNiO2 is formed when x is as close to 0 as possible. In practice, oxidizing all nickel atoms into Ni3+ during synthesis is challenging. Thus, LiNiO2 or nickel-rich layered oxides synthesized through modern methods usually possess at least ~ 1%–2% nickel atoms in their lithium layers [22]. Moreover, prolonged exposure to air and moisture easily results in deteriorating the performance of layered oxides. In particular, during shelf storage, the spontaneous reduction of Ni3+ into Ni2+ destroys the structural ordering of the material, causing LiNiO2 to undergo irreversible phase transformation during the intercalation/deintercalation of lithium ions.

Reproduced with permission from Ref. [3]. Copyright © 2019, American Chemical Society. c Effects of dopants on the properties, costs, and synthesis of LiNiO2. Reproduced with permission from Ref. [12]. Copyright © 2020, Springer Nature
a Schematic illustration of the crystal structure of LiTMO2. b X-ray diffraction (XRD) patterns of layered oxides with different Ni contents.
The strategy of substitution with various elements (e.g., cobalt, aluminum, or manganese) has been widely applied to obtain nickel-rich layered oxides NCA/NCM with increased stability. The substituent elements usually exert effects on the chemical structure of NCA/NCM upon lithium (de)intercalation to improve electrochemical performance. Figure 2c shows the effects of common dopants on the properties, costs, and synthesis of nickel-rich layered oxides [11, 12, 23, 24]. The incorporation of cobalt ions (3+) contributes to the ionic and electronic conductivity of the layered oxides. However, the capacity contribution from Co3+/4+ redox activity is limited due to structural failure induced by cathode overcharging. The incorporation of inexpensive manganese offers the advantages of mechanical robustness and thermal stability. However, manganese ions are electrochemically inactive and promote increased Ni2+ formation because of their charge neutrality, which deteriorates the electrochemical performance of the material. The functions of aluminum ions (3+) in the layered oxides are very similar to those of manganese ions (4+). Given that the atomic weight of aluminum is lighter than that of other transition metal elements, aluminum-doped nickel-based oxides have an increased gravimetric capacity. Furthermore, the very low Gibbs energy for the formation of Al2O3 increases the rigidity of the crystal structure. However, compared with its NCA counterpart, the nickel-based oxide NCM has inferior cycling stability due to active mass dissolution/crossover and irreversible phase transition [25].
2.2 Electronic Structure
Lithium deintercalation/intercalation from the layered structure during charge/discharge is closely related to redox reactions, which are essentially concerned with the outer electron gain/loss of TM ions. The electronic configuration wherein one TM ion coordinates with six oxygen atoms constitute a crystal field. As illustrated in Fig. 3a, the five 3d orbitals of transition metal elements are split into two sets, namely, the eg and t2g bands, in the crystal field produced by octahedral oxygen anion coordination [26, 27]. The lobes of the dx2–y2 and dz2 symmetries in the eg level point toward oxygen anions, whereas those of the dxy, dxz, and dyz symmetries in the t2g level point between oxygen anions. The interactive repulsion between oxygen anions confers eg levels with higher energy than the t2g levels.

Reproduced with permission from Ref. [26]. Copyright © 2017, John Wiley and Sons. d Local atomic environment around oxygen atoms in Li–O–TM configurations of stoichiometric layered nickel-based oxides. e Magnetic frustration of transition metal ion interactions. f Electron energy level illustrations of Mn, Ni, Co, and O ions
Schematic illustration of electronic structures. a Crystal field splitting of d orbitals in octahedral environments. b Typical electronic configuration for the Ni-rich layered oxide NCM. c Splitting of eg levels under Jahn–Teller distortion.
The commonly observed electronic configurations of the d orbital for elemental nickel, cobalt, and manganese in the nickel-based layered oxide NCM are presented in Fig. 3b. Given that Ni3+ and Mn3+ have a single electron at the eg level, they are stimulated by the Jahn–Teller effect at a low overall energy by breaking the degeneracy of two eg levels (Fig. 3c). The oxidation states of nickel, cobalt, and manganese have been clearly suggested to be 2+, 3+ and 4+, respectively, when they receive electrons from lithium during lithiation [28, 29]. After being fully charged, TM cations tend to be oxidized into the 4+ state to form TMO2. Thus, the chemical valence of manganese plays a vital role in stabilizing the crystal structure because it is unchanged. Figure 3d depicts the structure of layered oxides with the space group R3m. Transition metal atoms are arranged in a specific order in compounds with different antiferromagnetic structures and in other magnetic structures. Such results, for example, the relatively large possibility of arrangements in (333), (442), and (532), can change the cell parameters and total energy.
As indicated in Fig. 3e and f, long-range electronic structures are defined by magnetostatic interactions, whereas the band energy level can show the short-range structural features of a crystal field. The layered phase places TM ions at the vertex and center of the hexagonal ring in a TM slab. Thus, a two-dimensional triangular network is formed. The presence of unoccupied orbitals in the TM ions has an effect on the other surrounding ions: it always results in an increase in energy due to the sameness of the spin direction when the spin directions of the triangle sites are determined [30]. Figure 3f shows that in contrast to the 2p band of O, the 3d electron level splits into the eg and t2g energy bands of transition metal elements (nickel, cobalt, and manganese) [31]. Nickel-based layered oxides comprise a mixed valence state of 2+ and 3+. The overlapping area of the Ni3+/Ni4+ eg band and the top of the O2− 2p band is smaller than that of the Co3+/Co4+ t2g band and the top of the O2− 2p band. The Ni2+/Ni3+ eg band does not overlap with the O2− 2p band. These characteristics suggest that compared with cobalt, nickel, which has a higher valance state, has more chemical stability during charging [32]. The eg bands of Co3+/Co4+ and Mn3+/Mn4+ are considerably higher than the top of the O2− 2p band but are only oxidized in the high lithium deintercalation state at a high cutoff voltage [33].
3 Challenges of Nickel-Based Layered Oxides
3.1 Difficult Synthesis
The various methods and production processes of nickel-based layered oxides are shown schematically in Fig. 4a and b. The particle morphology, microstructure, and surface properties have a strong influence on the optimal energy output of layered oxides [34,35,36]. The overview of the main manufacturing procedures clearly shows that the synthesis route of nickel-based layered oxides needs precise control and production facilities that are capable of enduring harsh work conditions. Industry has deemed single-crystal cathodes with low and moderate nickel contents as commercially successful. Therefore, manufacturers have designed special equipment for the large-scale production of these cathodes (Fig. 4b). In commercial production, calcination and deagglomeration require highly sophisticated conditions, such as precise temperature and prolonged calcination time. Single-crystal materials are formed by eliminating the mechanical attractive force throughout the deagglomeration procedure. This approach is crucial for achieving the desired particle size and morphology. Commercial nickel-based layered oxides that consist of spherical secondary particles that are made up of hundreds of fine primary particles are usually prepared via the common coprecipitation method. The particle size and tap density of precursors are closely related to the experimental parameters. In the dissolution–recrystallization process, NH4OH, which is used as a common chelating agent, regulates the solubility of metal hydroxides and the growth of dense spherical particles. In accordance with the equilibrium and mass balances of all metal–ammonia complexes for nickel, manganese, and cobalt, nickel ions coordinate with ammonia at pH 4–12, whereas cobalt and manganese ions interact with ammonia in a narrow pH range of 6–10. Therefore, the crystallization and growth of nickel hydroxides are hard to control and require good equipment. The pH for nickel-rich precursors is typically 10–11. Qiu et al. [37] investigated the preparation conditions of nickel-based hydroxide precursors and designed end-point prediction modeling to optimize the pH, initial ammonia concentration, and metal sulfate addition. The calcination process for nickel-rich layered oxides is a complex problem.
Nickel and lithium ions are alternately aligned along the c-axis of the rhombohedral unit cell. The formation of the layered structure is mainly driven by the different sizes of Li+ and Ni3+, and the latter is oxidized from Ni2+ in the precursor. In fact, the crystal structure is a function of the oxygen chemical potential. Any unintended variation in μ(O2) may lead to decreased performance. Moreover, calcination causes lithium and oxygen loss that is accompanied by the formation of a rock salt phase [38] as a result of a chemical reaction during lithiation. It is regarded as the reverse of the synthesis process.
Compared with oxides with low nickel contents, nickel-rich oxides need a lower temperature, longer reaction time, flowing O2, and some special calcination equipment that has corrosion resistance against LiOH and O2. Li et al. [22] demonstrated the effect of calcination on the performance of nickel-based layered oxides. Their results indicated that the crystal structure and electrochemical performance were also influenced by the calcination parameters, such as the oxygen flow rate, sintering temperature, the initial Li/TM ratio, and sintering time. Posttreatment (e.g., washing, surface coating or element doping, and storage) after calcination is a very sophisticated process that has attracted considerable attention from researchers. Wang et al. [39] revealed that nickel-rich cathodes inevitably react with ambient air to form electrochemically inert Li2CO3 and LiOH. This behavior results in rapid capacity fading and high impedance when using nickel-rich cathodes. Although the nickel-rich layered oxides NCA/NCM may outperform LiCoO2 in terms of specific energy, they are less capable than LiCoO2 because of their lower electrode density.
3.2 Structural Instability
Upon intercalation/deintercalation from nickel-rich layered oxides, the host displays strong thermodynamic driving forces to undergo transformation from a layered to spinel structure via cation disordering and then undergoes transformation from spinel to rock salt via oxygen evolution [40, 41]. The relationship among layered, spinel, and rock salt structures is shown in Fig. 5a. The crystal structure of the layered lithium intercalation electrode is closely related to that of spinel and disordered rock salt. Within the same fcc oxygen framework, the arrangement of lithium and TM cations at octahedral and tetrahedral sites differs between spinel and rock salt. The TM ions present cubic symmetry when a quarter of them migrate from the TM layers to the octahedral sites in the lithium layers. The transformation from partly delithiated layered oxides into spinel structures becomes thermodynamically favorable as soon as a small amount of lithium is removed. The oxygen evolution reaction (OER) and abundant TM ions at the surface undergo regional densification, which leads to the degradation of the layered structure into rock salt. The rock salt structure wherein the TM layers and lithium layers no longer exhibit long-range periodic ordering presents an approximate TMO composition that is rich in transition metals.

Reproduced with permission from Ref. [26]. Copyright © 2017, John Wiley and Sons. b High-resolution transmission electron microscopy (TEM) and fast Fourier transformation (FFT) regions of the crystal structure changes that occur in NCM electrode materials after electrochemical processing, 1 Å = 1×10−10 m. Reproduced with permission from Ref. [43]. Copyright © 2014, John Wiley and Sons
a Relationship among the cation orderings of layered, spinel, and rock salt structures.
As described in Fig. 5b, the further extraction of lithium induces a mixed phase of layered and spinel phases in the core with the formation of the rock salt phase in the outermost surface [42,43,44]. In surface reconstruction, the low conductivity of the cubic phase can increase the impedance, and the phase transition is viewed as an undesirable process. However, similar to the solid electrolyte interphase (SEI) layer in the anode, the reconstruction layer can impede further reaction between the electrode and electrolyte by acting as a protective layer.
3.3 Chemical Instability
The OER is a major contributor to the structural instability of a cathode, reactions at the cathode/electrolyte interface, and safety problems. The correlation among gas evolution, the state of nickel oxidation, and the nickel content in cathode materials is shown in Fig. 6 [45]. The actual mechanism of the OER is unclear. Nevertheless, some arguments have been disseminated and are under discussion among researchers. Several active oxygen intermediates, such as covalent peroxide bonds (MO–OM), oxygen radicals (MO·), and excited-state singlet oxygen (1O2), have been verified by experiments (Fig. 6a) [47, 49, 50]. House et al. [51] demonstrated that oxygen loss is not triggered by vacancies in the TM layers and that high delithiation drives the electrode to a high degree of Li+ deficiency, thereby causing the local coordination to decrease to less than 3. Hwang et al. [42] proposed that the low energy barrier of electron migration from the O 1s state to the O 2p-Ni 3d hybridized state compromises the effective electron density of oxygen and is caused by increasing the reduction of Ni3+. Oxygen evolution in nickel-based layered oxides with any nickel content has been verified to occur at 75%–80% state of charge (SOC) (Fig. 6b), which is usually considered the stage of the H2–H3 phase transition [46, 47]. The rate of the OER accelerates with increasing SOC depth and operational temperature due to the thermodynamic instability of the H3 phase [44]. Nevertheless, the OER often occurs on the surface instead of in the bulk because of the kinetic hindrance of this reaction [48].

Reproduced with permission from Ref. [45]. Copyright © 2017, American Chemical Society
a Interfacial reactivity including surface reconstruction and CO2/O2 formation. b Dependence of the rate (CO2/O2) on the state of Ni oxidation. c Linear relationship between the state of Ni oxidation (SNOXi) and the Ni content in cathode materials. d Linear dependencies of the rising coefficient, χ, and rate (O2) data on the Ni/Co ratio.
For all nickel-based layered oxide composition, SNOXi decreases linearly as the Ni content increases (Fig. 6c), whereas both χ and γ(O2, lim) increase linearly with the Ni/Co ratio of oxides (Fig. 6d). The composition defines the electronic structure of the oxides. The removal of Li+, the reduction of nickel ions, and the high effective electron density of oxygen are thermodynamically contradictory, resulting in oxygen loss from the cathode to maintain charge balance. These fit-parameter dependencies explain the differences in the gas evolution, active oxygen formation, and surface reconstruction behavior of cathodes with varying composition.
The complex interfacial chemistry between the cathode and electrolyte is a knotty problem and plays a key role in the cycling life and safety of batteries. The electronic configuration at the TM sites is changed by the transfer of a hole from the TM 3d states to the highest occupied molecular orbital of the electrolyte [42, 52, 53], as shown in Fig. 7a. As evidenced by scientific experiments, the cathode electrolyte layer (CEI) in cathodes forms through the electro-oxidation of the electrolyte when an electrochemical test is performed or even when the cathodes interact with the electrolyte (Fig. 7b) [53, 55, 56]. The CEI is influenced by acid attack, carbonate electrolyte decomposition, and mass transfer between the active material and carbon black, wherein surface chemical composition involves many functional groups (e.g., hydroxyl, carbonyl, and carboxyl groups) (Fig. 7c) [54].

Reproduced with permission from Ref. [53]. Copyright © 2015, American Chemical Society. Schematic view of d ion transport and e resistance contributions in cells with electrode–electrolyte phase boundaries (SLEI, the solid-liquid electrolyte interphase; RE, the reference electrode; CE, the counter electrode; SE, the solid electrolyte; LE, the liquid electrolyte; gb, the grain boundary). Reproduced with permission from Ref. [56]. Copyright © 2016, Springer Nature
a Oxidation of TM ions occurs with the release of an electron from the host materials. b Hole transfer from the TM 3d states to the highest occupied molecular orbital (HOMO) of the electrolyte (LUMO: the lowest unoccupied molecular orbital). c Chemical composition of the CEI on a cathode.
A schematic of Li+ deintercalation in cathode materials is shown in Fig. 7d and e. Li+ transport across the interface is vulnerable to the properties of the CEI (solid electrolyte interphase). Capacity degradation and increased impedance are believed to be correlated with TM dissolution at high voltage [57,58,59]. Acid attack is the commonly accepted theoretical mechanism for TM dissolution. The oxidation of the electrolyte at high potential and the thermal instability of LiPF6 produce acidic species. Recently obtained experimental results have shown that TM dissolution occurs via a mechanism beyond simple HF attack [60]. The reduction of some TM ions, which is induced by the oxidative decomposition of the electrode/electrolyte and soluble TM complexes formed by cation exchange between the electrode and electrolyte, is likely the dominant mechanism for TM dissolution. Furthermore, the dissolved TM ions tend to migrate and deposit on the graphite anode and shorten the cycling life [25, 61].
3.4 Mechanical Degradation
The lithium intercalation/deintercalation behavior promotes obvious lattice structure distortion, resulting in the formation of strain, charge-state inhomogeneity, and crystal structural defects [62,63,64,65]. Compositional inhomogeneity and microcracks are responsible for the degradation of nickel-rich layered oxide materials. Figure 8 shows the changes in the a-axis lattice parameter, c-axis lattice parameter, and unit-cell volume as a function of cell voltage and the evolution of the normalized volume as a function of x in Li1–xMO2 [66]. At the same upper circuit voltage, the cell with a higher nickel content exhibits larger lattice parameters and more severe volume changes than the cell with a lower nickel content. These severe unit-cell volume changes always begin with the onset of the H2–H3 phase transition, which is an intrinsic property of nickel-rich layered oxides. Capacity fading and spherical aggregate pulverization are related to the sharp changes in parameters, which leads to the formation of cracks and promotes the penetration of electrolyte into the interface to contact a fresh surface, and the OER [67].

Reproduced with permission from Ref. [66]. Copyright © 2019, American Chemical Society
Changes in the a a-axis lattice parameter, b c-axis lattice parameter, and c unit-cell volume as a function of the cell voltage when using LiNi0.95Al0.05O2, LiNi0.95Mn0.05O2, LiNi0.95Mg0.05O2, LiNi0.9Co0.05Al0.05O2, LiNi0.8Mn0.1Co0.1O2, and LiNi0.5Mn0.3Co0.2O2 cathode materials. d Normalized volume as a function of x in Li1–xMO2.
As shown in Fig. 9a, in contrast to NCM92 (LiNi0.92Co0.04Mn0.04O2) at the initial charge state, NCM92 secondary particles that have undergone 100 cycles show obvious cracks that have propagated along the boundaries of primary particles from the center into the surface. After 100 cycles, numerous microcracks can be observed on all NCM96 (LiNi0.96Co0.03Mn0.03O2) secondary particles, whereas NCM92 shows some sparse, small hard lines. This apparent contrast between the microstructures of NCM92 and NCM96 suggests that higher nickel contents are prone to structural pulverization (called mechanical degradation). In addition, the resistance is directly proportional to the number of cycles for both composition, and the rate of increasing resistance of NCM96 exceeds that of NCM92 (Fig. 9b). Scanning spreading resistance microscopy images show the change in electronic conductivity between NCM92 and NCM96, thus confirming the existence of electronically dead regions (near-zero electronic conductivity) after the 100th cycle. The dead regions stretch along the microcracks, serving as the path for the penetration of electrolyte and promoting the buildup of the rock salt impurity phase (Fig. 9c). However, Ruess et al. [69] reported that microcracks improve the charge transfer kinetics and lithium diffusion of a material by expanding the electrochemically active surfaces and reducing the effective particle sizes.

Reproduced with permission from Ref. [68]. Copyright © 2019, American Chemical Society
a Cross-sectional images of Ni-rich layered oxides (percentage contents of Ni = 92%, 96%) after the 1st and 100th cycles. b Changes in the surface film and charge transfer resistance as a function of the cycling number. c Scanning spreading resistance microscopy (SSRM) images of NCM96 after the 1st and 100th cycles of full charge.
In addition to microcracks, the SOC heterogeneity in the secondary microstructure damages the cycling lifetime of nickel-rich layered oxides based on the lattice parameters and volume changes after (de)lithiation [70]. Random growth after coprecipitation results in the presence of a network of closed and open pores inside the secondary particles. Some physical factors, such as the primary particle alignment and local porosity, accommodate the parameter changes during electrochemical cycling. Consequently, the constant strain renders the secondary particles vulnerable to breaking into pieces, thus driving ions and electrons to distribute heterogeneously on a single-crystal material [71]. This physical change impedes the transport capability of the material and causes SOC inhomogeneity due to the overcharged regions of the single-crystal material at the cutoff potential [62].
3.5 Safety Problems
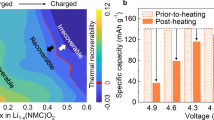
In addition to the above adverse effects of nickel-based layered oxides, safety problems pose an important challenge to the large-scale application of layered oxides. Thermal runaway caused by localized thermal accumulation already directly damages state-of-the-art LIBs during high-voltage operation at ambient temperature, let alone under harsh conditions [72, 73]. The safety problems are ascribed to the thermodynamic instability of the H2–H3 transition, leading to gas evolution (O2 and CO2) and heat generation. Figure 10a illustrates that thermal stability is inversely proportional to discharge capacity, which is closely related to the operating voltage, whereas the onset voltage of the H2–H3 transition decreases as the nickel content is increased. Figure 10b and c shows the high-resolution and low-resolution electron microscopy images of the widening and propagation of cracks during heating, respectively. Despite the application of an appropriate operating voltage, temperatures as low as 80–150 °C remain detrimental to layered oxide cathodes with high nickel contents because they promote structural evolution and Li/O loss [74, 75], as suggested by the schematic shown in Fig. 10d. This result is often believed to be a starting point for further catastrophe considering the intrinsic exothermic reaction of a layered oxide cathode and the insufficient dissipation in the case of rapid localized heat accumulation [76]. Various flammable components, such as the electrolyte solvent, conductive carbon, binders, and separators, in modern commercial LIB systems greatly accelerate a series of chain reactions until the entire battery combusts.

Reproduced with permission from Ref. [77]. Copyright © 2013, Elsevier. b Low-magnification image series showing crack propagation during heating. c High-magnification images from the selected region in (b) that show the structural changes and crack widening and propagation during heating. d Schematic illustration of the cracking process during heating. Reproduced with permission from Ref. [75]. Copyright © 2018, Springer Nature
a Map of the relationship among the thermal stability, discharge capacity, and capacity retention of varying composition.
Therefore, thermal runaway can still occur even without a short circuit, which includes the fracture of the separator and the formation of lithium dendrites [78]. Transition metal ions dissolve and migrate into the anode with an increase in the number of cycles. The transition metals that deposit on the anode can be used to analyze electrolyte decomposition, increase the thickness of the SEI layer and increase the consumption of lithium ions from the cathode. The cathode/anode ratio may continue to decrease after prolonged cycling. As such, an overcharged layered oxide cathode triggers safety issues even if charged to within the normal voltage range. Therefore, electric vehicles, especially those with aged batteries, are sometimes called invisible bombs.
4 Origin of Single-Crystal Nickel-Based Layered Oxides
As suggested in the above discussion, nickel-based layered oxides (NCM or NCA), derivatives of LiNiO2, are plagued by all kinds of problems. An unavoidable challenge encountered in these materials is the single-phase unit-cell contraction that is dependent on the degree of delithiation during charging/discharging. Mechanical strain caused by abrupt lattice distortion promotes the generation of microcracks in the structure of secondary particles and is believed to be responsible for cell failure, including the decreased interfacial reaction between the electrode and electrolyte, high impedance, and structural pulverization [3, 69, 70, 79,80,81]. Therefore, redesigning secondary particle structures and revisiting primary particles to address this fatal flaw may be the best ways to obtain long-term high-capacity cathode materials. Aggregations or agglomerations are made up of the smallest particles, which are called primary particles. In 2001, primary particles were defined as “the smallest identifiable subdivision in a particular system” by the National Institute of Standards and Technology [82]. The primary particles of nickel-based layered oxide cathode materials have been accepted in this field and are often considered single-crystal particles.
Many alternative approaches have been applied for the redesign of secondary particle structures. One such example is the core–shell gradient [83,84,85] and full concentration gradient [86,87,88] structures. A schematic of the concentration gradient and core–shell structures is shown in Fig. 11a, b. Studies have demonstrated that both structures improve the cycling stability and thermal stability of a material. Nevertheless, these structural designs have severe problems, such as large sacrifices in capacity and lattice mismatch generated by their varying composition during charging/discharging. Another microstructural modification example is the inducible orientation of primary particles. Elongated rod-like primary particles that grow radially from the center of the secondary particles are densely packed to alleviate the strain associated with the taxing H2–H3 phase transition (Fig. 11c).
a, b Schematic illustration of the full concentration gradient and core–shell gradient cathode materials. Reproduced with permission from Ref. [84, 88]. Copyright © 2009, 2012, Springer Nature. c Microstructure modified by radial texturing of primary particles. Reproduced with permission from Ref. [82]. Copyright © 2019, John Wiley and Sons. d Cross-sectional scanning electron microscopy (SEM) images and electrochemical performance of NCA89, NCM90, and NCB cathodes. Reproduced with permission from Ref. [89]. Copyright © 2020, John Wiley and Sons
Another emerging class of nickel-rich NCM and NCA cathodes has been developed through the use of dopants, such as boron [89], aluminum [34, 90], and tungsten [91]. In contrast to conventional dopants, these elements can be introduced to form a unique microstructure that suppresses the formation of microcracks and effectively improves the cycling stability of the material [92]. However, the microstructural modification of nickel-rich layered oxides with superior rate and cycling properties remains a challenge in regard to their industrial production.
The tuning of primary particles can facilitate lithium-ion transport, alleviate strain, and increase the mechanical strength of secondary particles. Even after nearly 30 years of the birth of LIBs, the single-crystal LiCoO2 that was first discovered by Prof. John B. Goodenough continues to dominate as the cathode material of LIBs in portable electronics due to its high compact density, long-term cycling ability, and thermal stability [1]. Dahn used accelerating rate calorimetry to compare the thermal stability of three Li0.5CoO2 materials with different particle sizes (diameters of approximately 0.8, 2, and 5 μm) and suggested that a large particle size is associated with a high self-heating onset temperature [93]. Commercialized LiNi0.6Co0.2Mn0.2O2 (NCM622) and LiCoO2 (LCO) with cathode loading levels of ~ 15 mg cm−2 and electrode densities of ~ 3.6 and ~ 3.8 g cm−3 for NCM622 and LCO, respectively, were subjected to comparative experiments under severe test conditions to develop the potential of nickel-rich cathode materials for practical applications [23]. The volume energy densities of NCM622 and LCO were identified by using a half-cell with voltage ranging from 3.0 to 4.3 V at 60 °C (Fig. 12a). LCO achieves good capacity retention with good cycling capability for 200 cycles, whereas NCM622 has a poor retention rate of ~ 5% after 200 cycles (Fig. 12b). The scanning electron microscopy (SEM) cross-sectional images of the cathode morphologies after the cycled cells were disassembled are shown in Fig. 12c–f. The electrochemically generated microcracks encompassing the cathode particles account for the capacity fading of NCM622. In contrast, the single-crystal LCO cathode particles remain intact. These results indicate that a single-crystal material outperforms a polycrystal material in terms of mechanical strength, cycling ability, and thermal stability.

Reproduced with permission from Ref. [23]. Copyright © 2018, John Wiley and Sons
a Volumetric energy density vs. the cycle number. b Voltage vs. volumetric energy density for commercialized NCM622 and LCO. SEM cross-sectional images of c, e the initial NCM622 and LCO cathodes and d, f NCM622 and LCO after 200 cycles at 60 °C.
In accordance with the professional classification (classical and nonclassical) of crystallization, the primary particles formed by the critical crystal nucleus exhibit uniform lattice orientation. Single-crystal nickel-based oxides are different from the single-crystal materials used in scientific research, wherein the smallest units (atoms, ions, and molecules) are orderly arranged in any solid object.
The orderly three-dimensional arrangement of atoms, ions, and molecules is repeated in any solid object. In contrast to single-crystal materials, polycrystal materials are composed of randomly oriented crystal regions called crystallites, which are formed when crystallization commences rapidly at many sites under supersaturated conditions. The structurally ordered regions growing from each site intersect each other. In layered oxides, secondary particles with a spherical morphology (~ 10 μm) are produced by energy industries, whereas single-crystal materials are the basic particles (< 1 μm) that form secondary spheres (Fig. 13). The metal ions and oxyhydroxide nanoparticles undergo continuous rotation and interaction until they find a perfect lattice match. Interfacial elimination proceeds at a rate that is consistent with the curvature dependence of the Gibbs free energy and is succeeded by lateral atom-to-atom addition initiated at the contact point [94]. Selected area electron diffraction and electron backscatter diffraction analyses performed in related works on electrode materials have verified that primary particles contain different crystal orientations and are thus not rigorously considered to be single-crystal materials. The relative crystal orientation of the grains is analyzed by comparing the orientation of the particles in a chosen frame.
Conventional strategies such as doping, surface coating, and microstructural modifications have been proposed by researchers to alleviate rapidly decaying capacities, but these methods fail to address these problems, as the methods usually require complicated technology and delicate control processes. In addition, they are not applicable to large-scale production. With the good cycling and thermal stability of single-crystal LiCoO2, a single crystal of nickel-based layered oxides was developed. Specifically, our version of a “single crystal” is clearly different from the single-crystal definition in theoretical terms and is used to describe layered oxides from the perspective of morphology. According to multiple studies, single-crystal layered oxides demonstrate superior performance to polycrystal oxides. Compared to other modifications, the production technique of single-crystal oxides is relatively controllable, and single-crystal oxides with moderate nickel contents can be obtained from the market for use as cathode materials.
5 Synthesis of Single-Crystal Nickel-Based Layered Oxides
5.1 Synthesis Methods
The most economical synthesis route of single-crystal cathode materials is the simple controlled-temperature calcination of coprecipitated precursors with particle sizes that are usually smaller than the industrial norm (3–4 μm versus \(\geqslant\) 10 μm). The crucial component of this method is the appropriate synthesis temperature, which is dependent on the different nickel contents of the nickel-based layered oxide cathode materials. In the calcination process, high temperatures (usually defined as \(\geqslant\) 850 °C) result in extensive fracture surfaces and large amounts of fine particles in the cathode material (Fig. 14a, b, unpublished results). Subsequently, an increase in the exposed interface between the cathode and electrolyte results in further side reactions and gas evolution. In addition, nickel-rich cathode materials are prone to oxygen and lithium-ion loss under high-temperature conditions, resulting in the formation of electrochemically inactive phases, such as the rock salt phase NiO [95]. As suggested in Fig. 14c, high temperatures induce the domination of Li+/Ni2+ cation disordering on the structural evolution of nickel-rich cathodes; this phenomenon implies transformation from a layered structure to a rock salt structure and capacity degradation [95, 96].

Reproduced with permission from Ref. [97]. Copyright © 2012. American Chemical Society. c Schematic illustration of the phase evolution of intermediates and the slab distance of the Li and Ni (Co) slabs as a function of temperature. Reproduced with permission from Ref. [95]. Copyright © 2016, John Wiley and Sons
a, b SEM images of the NCM after calcination at 900 °C, which show many small particles and fractures.
Given the morphological and structural defects induced by high temperatures, synthesizing single-crystal nickel-rich cathodes at temperatures lower than 950 °C by using the low mutual melting eutectic of molten salt or flux growth methods is necessary. In contrast to solid-state reactions at high temperatures, flux growth is well known as a liquid-phase method that provides high-quality, well-developed oxide crystals at low temperatures. Several factors positively influence the capability of flux to promote crystal growth [98]: (1) substantial reagent solubility, (2) low toxicity, (3) high chemical stability, (4) unreactive to containers, (5) easy removability, (6) variable solubility with parameter changes, and (7) low commercial cost and easy acquisition. Three sequential processes—dissolution, nucleation, and crystal growth—constitute the crystal formation mechanism. The growth rate of particles is closely related to the degree of supersaturation. Excessively high supersaturation results in numerous crystalline sites and further dendrite growth rather than new crystal growth with good facets, whereas excessively low supersaturation prevents nucleation altogether. Thus, identifying the optimal supersaturation point is crucial to acquire high-quality single-crystal materials. In the synthesis of single-crystal nickel-based oxides, the application of the appropriate flux combined with the cathode precursor results in the formation of an even lower melting eutectic wherein the fine particles comprising the precursor dissolve and recrystallize, resulting in the synthesis of narrow single-crystal materials. Furthermore, the ideal outcome of a uniform single-crystal size is closely tied to the relative rates of nucleation and growth and thus requires excellent temperature control.
Recently, some representative molten salts, including KCl [97, 99], NaCl [97], Li2MoO4 [100], LiOH–LiNO3 [101], LiCl–KCl [102], NaOH [103], LiNO3 [104], KMnO4 [105], Li2SO4 [106], Na2SO4 [107], and Li2CO3 [108], have displayed good fluxes to favor crystal nucleation and growth at comparable temperatures for single-crystal layered oxide cathodes. The morphological features of the crystals are controlled not only by the structure of the reagent itself but also by the nature of the chemical fluxes. In other words, the growth rate along the different facets of a single-crystal material highly determines the formation of a crystal habit based on the interactive physicochemical property of the preferred facet [109]. For example, a crystal with a cubic structure can become either a cube or an octahedron. A cube forms if the growth rate along the (1 0 0) facet is the fastest, whereas an octahedron forms if the growth rate along the (1 1 0) facet is the fastest. Furthermore, given that the presence of anions and cations in the flux can influence the growth conditions of reagents, selecting the appropriate flux by matching physical and chemical properties is beneficial to the formation of high-quality single-crystal materials [110]. The flux method usually involves follow-up processes, such as breaking and classification sieving. In addition to the previously discussed factors, the easy removal of residual chemical agents introduced by the flux growth method via dissolution in common solvents, ideally water, is an important factor for good flux. However, layer oxides with high nickel contents are vulnerable to damage caused by atmospheres containing H2O and CO2 [111, 112]. Research suggests that washing can be used to remove residual surface salts, and superficial properties are modified by Li+/H+ exchange behavior [113, 114]. Consequently, this flux growth method does not conform to the requirements of industrial production in terms of time and cost.
Therefore, the limitations of the flux growth method have driven researchers to develop numerous other strategies to synthesize highly pure single-crystal materials. Several methods, such as the hydrogen peroxide-assisted coprecipitation [115], sol–gel method [116,117,118,119], template method [120], liquid-phase method [121,122,123], and hydrothermal method [124,125,126], have been reported to provide nanocrystal layered oxides rather than the secondary particles produced by the coprecipitation process. For example, Lin et al. [115] reported that LiNi1/3Co1/3Mn1/3O2 with a single-crystal structure and good battery performance can be skillfully prepared through a coprecipitation approach by first utilizing H2O2 as the additive and then performing ozone-assisted thermal posttreatment (Fig. 15a). H2O2 not only performs as a strong oxidant but also acts as a simple dispersant that can decompose and release O2 to disperse precursors via coprecipitation. The hydrothermal synthesis of single-crystal precursors is also a common method for fabricating materials with controlled morphology and size. For example, uniform, well-crystallized single particles with diameters of approximately 800 nm are synthesized at a hydrothermal temperature of 200 °C (Fig. 15b), as reported by Wang et al. [4] Zhang et al. [125] synthesized hollow hierarchical Ni0.8Co0.1Mn0.1CO3 precursors under hydrothermal conditions. Fu et al. [119] fabricated LiNi1/3Co1/3Mn1/3O2 hexagonal nanobricks with approximately 58.6% exposed (0 0 1) facets through the precursor–template method. These nanobricks exhibit good rate performance in LIBs. The surface-capping agent poly(vinylpyrrolidone) plays an important role in the solution system. It adsorbs on negatively charged (0 0 1) surfaces via the amine group to reduce the growth rate along the [0 0 1] direction (Fig. 15c). Zhu et al. [123] first adopted the spray pyrolysis method to synthesize a microsphere precursor and then adjusted the sintering temperature and lithium source to obtain single-crystal NMC811. Wu et al. [118] proposed the preparation of single-crystal LiNi0.32Mn0.33Co0.33Al0.01O2 materials through the sol–gel method with citric acid as the chelating agent (Fig. 15d).

Reproduced with permission from Ref. [114]. Copyright © 2015, Elsevier. b Reproduced with permission from Ref. [4]. Copyright © 2016, Elsevier. c Reproduced with permission from Ref. [119]. Copyright © 2013, Royal Society of Chemistry. d Reproduced with permission from Ref. [117]. Copyright © 2009, Elsevier
SEM images of single-crystal nickel-based cathodes synthesized by different methods. a
Figure 15 shows four representative single-crystal morphologies fabricated via different synthesis methods. Although these methods can produce highly pure single-crystal cathodes with diameters of less than 1 μm without the use of high temperatures, the power tap/pellet density is too low to reach the requirements of a cathode with a high energy density. In addition, from a practical viewpoint, methods involving volatile and toxic organic/inorganic solvents are contradictory to large-scale production. Single-crystal nickel-based layered oxides with different morphologies and synthesized by using various methods are summarized in Table 1. Flux growth remains a common synthesis method for different nickel-based layered oxides with varying composition because it enables the easy removal of impurities and is a simple procedure.
The use of single-crystal nickel-based layered oxides as cathode materials has notable progress due to the motivation of achieving superior properties and the various preparation methods for single-crystal materials. In this section, several important developments of single-crystal cathode materials are introduced, evaluated, and summarized.
Zhu et al. [133] investigated the growth behavior of the layered oxide LiNi1/3Co1/3Mn1/3O2 prepared by utilizing coprecipitation and high-temperature calcination. Particle morphology is highly dependent on the synthesis method and annealing procedure, which control the packing density, diffusion rate, and pathway. The SEM images in Fig. 16a–f show the relationship between the particle morphology and temperature. The crystal diameter as a function of different temperatures is exhibited in Fig. 16g, and the corresponding crystal growth mechanisms have been established by researchers (Fig. 16h). At temperatures less than 750 °C, grain growth is minimal, and the rate-controlling exponent is low and dependent on surface diffusion. As the temperature is increased to 800–900 °C, volume diffusion is expected to become the dominant growth mechanism. Crystal growth can be controlled by grain boundary diffusion over the temperature range of 900–1 000 °C. This investigation provides a theoretical path for synthesizing single-crystal nickel-based oxides via solid-state calcination.

Reproduced with permission from Ref. [133]. Copyright © 2012, American Chemistry Society
SEM images at different temperature: a 600, b 700, c 800, d 900, e 950, and f 1 000 °C for 3 h. g Crystal diameter distribution for the temperature range from 600 to 1 000 °C. h Functional relationship between ln(crystal diameter) and ln(time).
Kim et al. [97] reported on the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2 as an LIB cathode, which was synthesized by using alkali chlorides as a flux. Flux growth using alkali chlorides lowers the heating temperature in the solid-state reaction to reduce unstable fracture surfaces and eliminate fine particles that degrade electrode performance. The schematic in Fig. 17a shows that different fluxes directly influence the primary morphology and promote the exposure of specific crystal facets. The surfaces of particles grown with KCl flux include additional (0 1 2) and equivalent facets, whereas well-developed (0 0 3) and (1 −1 −1) equivalent facet planes are obtained in the presence of liquid NaCl. Figure 17b illustrates that flux growth can separate the primary particles of agglomerates and expedite crystal growth. In addition, flux growth can be used as an effective method for reducing gas emission by controlling the interfacial area between cathode materials and electrolytes and providing increased volumetric capacity. Kimijima et al. [100] proposed using Li2MoO4 as a flux to fabricate NCM crystals that form polyhedral shapes with (1 1 3), (0 1 1), (1 0 2), and (2 1 3) facets. In the Li2MoO4 flux approach, the morphology and particle size can be controlled by tuning the solute concentration, holding temperature, and cations in the molybdate flux. Zhu et al. [99] systematically studied the effect of the nickel content, grain size, and crystal facets on the chemical stability and electrochemical performance of the material. They correlated the properties and high-voltage performance of nickel-based oxides by synthesizing single-crystal materials under different conditions as a study base. The initial experiment showed the evolution of the crystal morphology as a function of the oxygen and lithium chemical potentials and the influence of the (0 1 2), (0 0 1), and (1 0 4) facets on the surface (Fig. 17c) and verified previous theoretical calculations. As shown in Fig. 17d–g, NCM samples with different morphologies can be synthesized by regulating the oxygen and lithium chemical potentials.

Reproduced with permission from Ref. [97]. Copyright © 2012, American Chemistry Society
a Schematic illustration of single-crystal nickel-based cathodes with truncated polyhedral shapes during the crystal growth process. b SEM images of materials after using KCl and NaCl fluxes at different temperature (800, 900, and 1 000 °C). c Evolution of the crystal shape as a function of the O and Li chemical potentials and influence of the (0 1 2), (0 0 1), and (1 0 4) facets on the surface. SEM images representing the d octahedral-shaped, e truncated octahedral-shaped, f polyhedral-shaped (poly), and g platelet-shaped structures of the same NCM crystal sample.
Qian et al. [106] synthesized microsized single-crystal nickel-rich oxides via an industrially applicable flux growth method. Fracture mechanics theory has verified that in contrast to polycrystals, single crystals eliminate the risk of intergranular fracture due to electrochemical cycling induced by coherency stress [134]. Analysis through fracture mechanics calculations revealed that single-crystal particles must have an average critical size of ~ 2 μm to be fracture resistant. Even though single-crystal materials also experience phase transformation after lithiation/delithiation, this transformation improves capacity retention by eliminating frail internal grain boundaries and intergranular fractures, as illustrated in Fig. 18a–e. Figure 18f–h confirms the layered structure of the single-crystal cathode materials prepared by the molten salt-based method. Moreover, a well-defined phase with a surface cation-disordered phase that is 3–5 nm thick suggests that the single-crystal oxide structure prepared by the molten salt-based high-temperature method is prone to transform from a layered structure to a rock salt phase. The cross-sectional SEM images of single-crystal and polycrystal materials polished through argon-ion milling are provided in Fig. 18i–l. Single-crystal NCM622 shows no signs of microcracks after 300 cycles, whereas its polycrystal counterpart has clearly cracked along its grain boundaries, as was also reported by Fan et al. [135]; this result is consistent with previous reports on polycrystal particles [68, 79, 81]. A rough estimate shows that the primary particle size (single-crystal material vs. polycrystal material: 2 μm vs. ~ 200–500 nm) is inversely proportional to the capacity loss due to surface reconstruction.

Reproduced with permission from Ref. [106]. Copyright © 2020, Elsevier
a, e SEM image and mapping images of the as-synthesized single-crystal NCM622. b XRD pattern and the Rietveld refinement results. c Band contrast electron backscatter diffraction (EBSD) map and d EBSD orientation map of the particles. f Scanning transmission electron microscopy (STEM) images of a particle oriented along the [0 0 1] zone axis. g, h High-angle annual dark-field STEM (HAADF–STEM) images showing a good layered structure in the interior and a mixed-cation layer on the exterior surface. i, j Cross-sectional SEM images of single-crystal NCM622 before cycling and after 300 cycles. k, l Cross-sectional SEM images of polycrystal NCM622 before cycling and after 300 cycles.
Several works on single-crystal positive material synthesis through the molten salt method have been elaborated above. However, the challenging follow-up handling and fabrication cost of this method have been problematic and unacceptable for mass production. Dahn et al. have made major contributions to the development of single-crystal nickel-rich layered oxides. Li et al. [128] synthesized single-crystal NMC532 through a simple solid-state calcination method and found that the lithium/precursor ratio and temperature during synthesis played vital roles in its structure and morphology. Excess lithium can provide a self-flux environment that facilitates particle growth [136], while high temperatures can meet the energy requirements of particle growth. The optimal synthesis conditions should be based on a balance among the grain size, reversible and irreversible capacity, and rate capacity. Single-crystal NMC532 with a grain size of 2–3 μm was compared with conventional uncoated polycrystal NMC532 and Al2O3-coated polycrystal materials [137]. The single-crystal and polycrystal NMC532 samples are shown in Fig. 19a. Figure 19b depicts the detected intensity of the oxygen that evolved from the three samples at 4.2, 4.4, and 4.6 V. Figure 19c displays the normalized capacity of single-crystal NMC532 and coated polycrystal NMC532 tested at 40 and 55 °C. The experimental results suggest that single-crystal NMC532 has considerably better stability against electrolytes at high voltages (4.4 V and above) and higher temperature than the coated and uncoated polycrystal materials. Dahn et al. [129, 130] synthesized single-crystal NMC622 and LiNi0.88Co0.09Al0.03O2 by using nearly the same method. The effect of electrolyte additives on the performance of a single-crystal material has been discussed, and surface area measurements have been used to rule out the opinion that a single-crystal material has a small surface area and thus fewer side reactions with electrolytes. Instead, a single-crystal material benefits from its particle integrity and SEI stability. Furthermore, single-crystal NMC622 with a large grain size shows inferior rate capacity. Therefore, work related to improving the rate capability of these single-crystal materials may be valuable.

Reproduced with permission from Ref. [137]. Copyright © 2017, The Electrochemical Society
a SEM images of a single-crystal NMC532 with a large grain size of ~ 3 μm and polycrystal NMC532 with a small grain size of ~ 250 nm. b Detected oxygen intensity evolved from the three samples at 4.2, 4.4, and 4.6 V. c Normalized capacity of single-crystal NMC532 and coated polycrystal NMC532 as a function of the cycle number and tested at 40 and 55 °C, respectively.
Several synthesis methods for single-crystal oxide cathode materials have been explored. The vapor-phase deposition method, wherein atoms leave the gas phase and deposit on surfaces at rates faster than their departure, is used to synthesize crystals with specific surface arrangements. However, this synthesis method has some limitations: (1) the compound or monomer has to have a high vapor pressure, (2) complex equipment is required, and (3) a low output is obtained. Large single crystals may be easily grown in a liquid–solid system, wherein approximately 20%–30% of the desired solute dissolves into the solvent and forms a stable solution. The solubility of the solute varies in accordance with the change in parameters, such as temperature, pH, or pressure.
Figure 20 illustrates the different formation mechanisms of various solids. In the absence of crystallization, precursor ions with disordered arrangements on the atomic scale condense and form amorphous solids, such as the hydroxide precursor of the cathode material (Fig. 20, precursor ions → amorphous solid). Crystal materials usually include single-crystal, polycrystal, and mesocrystal materials [136]. Single-crystal layered oxides for cathode materials are formed nonclassically in a calcination process wherein crystal growth typically proceeds via the integration of classically crystallized subunits. In particular, the nanocrystals of hydroxide precursors attach to each other in an oriented manner via mesoscale assembly (Fig. 20, precursor ions → nanocrystal → mesocrystal). Mesocrystals consist of nanocrystals in which the crystallography is completely aligned. In fact, high supersaturation usually gives rise to abundant crystallites that are highly unstable due to high surface/volume ratios. Polycrystal formation can effectively relieve high surface energy by randomly aggregating in the absence of crystallographic alignment (Fig. 20, precursor ions → nanocrystal → polycrystal). Thus, the primary particles of nickel-based layered oxides are not true single-crystal materials. Classically, the formation of a single-crystal material requires a dedicated crystallization process starting from the assembly of primary building blocks, such as atoms, ions, and molecules. In a typical Ostwald ripening process, crystallization commences at many sites. The crystals reach a critical size and then intersect each other for further growth (Fig. 20, precursor ions → nanocrystal → single crystal). The growth of large crystals at the expense of small crystals is driven by thermodynamic forces.
The prominent method to prepare single-crystal oxides is still flux growth. The eutectic point between different salts not only helps lower the calcination temperature but also promotes the growth rate of primary particles. Other methods, such as the hydrothermal and sol–gel methods, are underdeveloped because their uncontrollable technical parameters result in irregular shapes and sizes of single-crystal materials under different operational conditions, which can have a large impact on their electrochemical behavior. Single-crystal oxides with preferred crystal faces and significant electrochemical performance should be synthesized through specific regulations.
The synthesis methods of layered oxides become more challenging with higher nickel contents, since a higher nickel content means the material is more sensitive to temperature and more vulnerable to structural defects. With further research, it is believed that the era of large-scale production is coming soon.
6 Research Comparing Single-Crystal and Polycrystal Materials
Only a few reports have demonstrated that single-crystal cathode materials are superior to their polycrystal counterparts in terms of electrochemical stability [4, 105, 126, 128, 134, 137,138,139]. Weber et al. [140] aimed to further understand the cycling behavior of single-crystal and polycrystal LiNi0.5Co0.2Mn0.3O2 through operando X-ray diffraction. They revealed that the lattice parameters and unit-cell volume of the two cathode materials exhibited identical trends of variation during cycling (Fig. 21a, b). This result proved that the outstanding performance of NMC532 could not be attributed to differences in the lattice parameters during lithiation and delithiation and that some other factors were responsible. Li et al. [141] proposed appropriate electrolytes for single-crystal NMC532/artificial graphite cells exhibiting excellent long-term cycling lifetimes at 4.4 V and elevated temperature. Electrochemical characterization techniques, such as in situ gas evolution, ultrahigh precision coulometry, isothermal microcalorimetry, and lithium plating, as well as long-term cycling tests, have been applied to screen electrolytes [141,142,143]. Electrolytes containing 2VC + 1DTD or 2FEC + 1DTD as additives are good candidates for single-crystal NMC532/graphite cell systems that exhibit long cycling lifetimes and C-rate charges at room temperature. Liu et al. [144] collected cross-sectional SEM images of single-crystal LiNi0.5Co0.2Mn0.3O2, LiNi0.6Co0.2Mn0.2O2, and LiNi0.8Co0.1Mn0.1O2 from commercial-grade pouch cells that were heavily cycled at 4.3 V (Fig. 21c–e). The SEM images revealed very little sign of the microcracks that were responsible for capacity degradation. Moreover, as a result of electrode calendaring, the cycled electrode showed a visible difference that was negligible from the fresh electrode. This result further proved that single-crystal materials have increased robustness against microcracks and thus contributes to an excellent capacity retention when used in LIBs. He et al. [145] recently investigated the effects of substituting cobalt with nickel on the structures, morphologies, and electrochemical properties of single-crystal LiNi0.6+xCo0.2–xMn0.2O2 (0.0 \(\leqslant\) x \(\leqslant\) 0.1). Similar to those of its polycrystal counterpart, the cycling performance and thermal stability of the single-crystal material gradually deteriorated as the nickel content was increased and the cobalt content was decreased because of the increased degree of cation mixing and resistance.

Reproduced with permission from Ref. [140]. Copyright © 2017, The Electrochemical Society. Cross-sectional scanning electron microscopy (SEM) images of single-crystal c LiNi0.5Co0.2Mn0.3O2, d LiNi0.6Co0.2Mn0.2O2, and e LiNi0.8Co0.1Mn0.1O2 from heavily cycled commercial-grade pouch cells at 4.3 V, respectively. Reproduced with permission from Ref. [144]. Copyright © 2020, The Electrochemical Society
a Operando X-ray diffraction data of the polycrystal and single-crystal NMC532 cathode. b Lattice parameters and cell volume as a function of potential are shown.
Furthermore, a single-crystal material shows high promise for alleviating battery safety issues. Single-crystal cathodes alleviate gas evolution (particularly oxygen) by preventing the microcracking that is induced by electrochemical cycling and side reactions with the electrolyte. During storage at a fully charged state (4.45 V) at high or ambient temperature, single-crystal LiNi0.8Co0.1Mn0.1O2 exhibits less gas evolution than the commercial polycrystal NMC532 cathode at high temperature (85 °C), as shown in Fig. 22a [97]. Figure 22b shows the gas volume of single-crystal NMC532 and Al2O3-coated polycrystal NMC532 at high voltages for 300 h [137]. The single-crystal material produces a negligible amount of gas, whereas the polycrystal material produces considerably higher amounts of gas. This result indicates the superior stability of single-crystal materials.

Reproduced with permission from Ref. [97]. Copyright © 2012, American Chemistry Society. b Gas volume of single-crystal NMC532 and Al2O3-coated polycrystal NMC532 as a function of time. Reproduced with permission from Ref. [137]. Copyright © 2017, The Electrochemical Society. c Plot of the normalized capacity and V/Vo versus time of NMC532/graphite cells undergoing cycling or storage testing at 20, 40, and 55 °C. Reproduced with permission from Ref. [146]. Copyright © 2019, The Electrochemical Society
a Gas evolution of charged NCM811 and commercial NCM532 at 85 °C.
Many researchers have reported that the electrochemical performance of a single-crystal material is superior to that of other morphological crystals. Dahn’s group [146] presented a wide range of testing results for single-crystal NMC532/graphite pouch cells for LIBs with moderate energy density. In the report, two failure mechanisms, lithium inventory and impedance growth, dominated the lifetime of cells. Figure 22c shows the normalized capacity and ∆V/Vo for NMC532/graphite full cells undergoing cycling or storage at 20, 40, and 55 °C. The impact of cycling and storage on the electrochemical lifetime are compared. The increasing rates of impedance, which is indicated by ∆V/Vo for cells undergoing charge–discharge and those undergoing storage testing, are very similar. The charge–discharge test sample exhibits slightly more capacity loss than the storage test sample per unit of time. Regarding cells undergoing cycling or storage testing at 55 °C and cells undergoing cycling at 20 and 40 °C, capacity loss is attributed to loss of the lithium inventory due to a thickening SEI on the negative electrode. Furthermore, this type of pouch cell should be capable of powering an electric vehicle for over 1.6 million km and last for at least two decades in grid energy storage. This single-crystal cathode exhibits great potential for use in commercial applications and serves as a benchmark for academics and industries developing advanced LIBs.
After reviewing many studies, a summary of the single-crystal and polycrystal materials are compared through the use of radar maps that include the tap density, synthesis complexity, thermal stability, rate performance, cycling stability, and cost (Fig. 23). It is widely believed that the overall performance of single-crystal materials with different nickel contents is superior to that of polycrystal materials, which has been proven by many reports, especially in regard to the cycling performance. However, considerable arguments still exist, including the thermal stability, tap density, and safety of single-crystal oxides. If single-crystal nickel-rich layered oxides are commercialized extensively, many parameters other than electrochemical performance should be standardized by researchers.
7 Recent Processes for Single-Crystal Nickel-Based Cathode Materials
7.1 Doping and Surface Coating
Many strategies are also used to enhance the capacity and thermal stability of polycrystal materials (secondary particle level) to expand their predominance over a wide temperature range and at a high cutoff potential. The most prominent and conventional strategies include atom doping and surface coating. Atom doping implies the substitution of atoms in the crystal lattice (nickel, cobalt, and manganese sites) with stable dopants, such as calcium [147], magnesium [148,149,150,151], aluminum [152,153,154,155,156,157], titanium [158, 159], zirconium [160], molybdenum [161], tungsten [160], niobium [162], and tellurium [163]; doping with these elements reinforces the structural integrity of the material to mitigate the tension brought by the phase transition during lithiation and delithiation [164]. Regarding surface coating, these dopants all act as a protective layer to prevent the cathode materials from coming into contact with the electrolyte, thus suppressing parasitic side reactions between the cathode and electrolyte. For example, oxide coatings [165,166,167] can react with HF to preserve the cathode against corrosion, while fluoride [168,169,170,171] and phosphate [172,173,174] coatings are formed as a thermodynamically stable layer after the initial cycles. In fact, these conventional strategies merely delay the onset of cathode degradation. Mechanical stress continues to build at the grain boundaries until the secondary hierarchical structure is pulverized, resulting in capacity loss and safety problems. Nonetheless, the capability of a single-crystal material without grain boundaries to withstand strain can be improved. Therefore, modifications via conventional strategies may considerably contribute to the performance of single-crystal materials. A few modification processes centering on single-crystal nickel-based layered oxides are discussed below.
Zeng et al. [175] synthesized surface aluminum-doped single-crystal NMC532 by using a combination of coprecipitation and calcination. The aluminum contents of the surface and bulk of the product are approximately 1.05% and 0.02%, respectively. NMC532 with an Al-doped surface shows improved capacity retention at high temperature and an upper cutoff voltage because of its stable interfacial structure. Zhao et al. [176] revealed that fluorine-doped single-crystal nickel-based cathode materials promote the expansion of the unit cell along the c-axis. This phenomenon is conducive to the kinetics of Li+ transportation. The calculated lithium-ion diffusion coefficient (DLi+) indicates that the migration of lithium ions is smoothed, thus improving the electrochemical performance of cathode materials. Zeng et al. [177] proposed forming multifunctional LiBO2/LiAlO2 layers on the surfaces of NMC532 by using a surface coating strategy. The LiAlO2 layer sustains intimate contact with the cathode, while LiBO2 acts as an external isolation layer to prevent interfacial instability between the cathode and electrolyte. This surface coating may also improve electrochemical performance.
7.2 Mechanical Study
Beyond doping and surface coating, the particle morphology, interfacial properties, and application of single-crystal cathode materials have been preliminarily investigated. Zhang et al. [178] discussed the relationship between the particle size and electrochemical properties of NMC811 obtained through the solid-state method. Experiments showed that particle sizes changed potential polarization by controlling the capability to transfer lithium ions and charge. Zhang et al. [181] aimed to understand the underlying deterioration mechanism of single-crystal materials by using operando synchrotron X-ray spectroscopic microscopy and X-ray nanotomography. Figure 24a provides the operando qualitative chemical mapping for the initial and 201st cycles. As observed in this image, approximately half of the phase composition cannot recover to a pristine state, and the single-crystal NCM exhibits a highly heterogeneous phase distribution. As mentioned above, Zhu et al. [99] established the effect of variable physical parameters on the surface stability and electrochemical performance. NMC cathodes dominated by (0 0 1) or (1 0 4) facets show better high-voltage cycling performance than samples with more (0 1 2) facets. The (0 1 2) facets are generally more reactive with electrolytes than the (0 0 1) facets, as evidenced by their increased reduction of surface nickel and higher initial capacity but poorer capacity retention. Another study reported by Zhu et al. [179] focused on the composition- and facet-dependent surface reconstruction behavior of pristine and cycled particles. Figure 24b shows the formation and growth of the surface reconstruction layer on (a, up) pristine and (a, down) cycled particles. During synthesis, an increasing annealing time and annealing temperature may lead to lithium and oxygen loss on the non-(0 0 1) surface with the open lithium diffusion channel [180]. Vacant lithium sites are easily occupied by nickel cations to form antisite defects. In accordance with the degree of Li/O loss, the surface densification and reconstruction layer on the non-(0 0 1) surface may form spinel and rock salt phases. In addition, delithiated NCM is thermodynamically less stable than its pristine counterparts when lithium ions are extensively extracted at high voltage during the electrochemical process. The low energy barrier of interplane transport promotes nickel enrichment and the growth of the reconstruction layer. Surface engineering opens a new avenue for the tradeoff between capacity and stability, especially for single-crystal cathode materials. Single-crystal cathodes are no exception. The degradation of single-crystal NCM itself still occurs at high cutoff voltages. After demonstrating that the surface rock salt phase is effectively detrimental to lithium transport, surface modification has been conducted by using a feasible lithium source, oxygen flow, and appropriate temperature to replenish lattice sites. Surface chemistry regulation is verified to be a valid way to develop advanced single-crystal battery materials. All-solid-state LIBs with high energy density and reliable safety have attracted considerable attention in recent years. Wang et al. [182] explored the electrochemical performance of single-crystal cathode materials matched with solid-state sulfide electrolytes for the first time. Their experimental and theoretical verification of electrochemical performance showed that the lithium diffusion coefficient of single-crystal NMC532 is 6–14 times higher than that of polycrystal NMC532 with many grain boundaries. The cycling and rate performance of all-solid-state LIBs with single-crystal cathodes are superior to those of their polycrystal counterparts.

Reproduced with permission from Ref. [181]. Copyright © 2020, Springer Nature. b Atomic-resolution scanning transmission electron microscopy (STEM) images and corresponding schematic illustration showing the reconstruction layer on the (0 0 1) and non-(0 0 1) surfaces before and after cycling. Reproduced with permission from Ref. [179]. Copyright © 2020, American Chemistry Society. c Diffraction pattern of electrochemically aged single-crystal NCM after 500 cycles at the charged state (4.3 V vs. Li/Li+). Inset: the expanded (003) reflection region to illustrate peak splitting. Reproduced with permission from Ref. [183]. Copyright © 2020, Springer Nature. d Atomic force microscopy images of the morphology and surface structure at the open-circuit voltage. Reproduced with permission from Ref. [184]. Copyright © 2020, American Association for the Advanced of Science. e Kinetic limitations of a single-crystal material against a polycrystal material when charging during a finite element analysis simulation. Reproduced with permission from Ref. [185]. Copyright © 2020, John Wiley and Sons
a Operando 2D chemical phase mappings and quantification bar charts at the nickel K-edge of NMC particles at the 1st and 201st cycles.
Xu et al. [183] proposed that the lattice mismatch between the layered structure and rock salt surface reconstruction causes fatigue degradation in nickel-rich oxides (Fig. 24c). These fatigue regions are not attributed to the intergranular cracking and kinetic limitations that still occur in layered oxides. Recently, Xiao et al. [184] observed reversible planar gliding and microcracking along the (003) plane in a single-crystal nickel-rich cathode. The concentration gradient of Li+ in the lattice caused localized stress, thus further inducing microstructural defects in the nickel-rich oxides. Figure 24d shows the surface structure and morphology evolution at the open-circuit voltage. The origin and evolution of gliding steps and microcracks in an electric field have been probed kinetically during charge and discharge. Wang et al. [185] revealed that the strong dependence of redox kinetics on the SOC indicated that the redox reaction was sluggish at low SOC and increased rapidly as the SOC increased (Fig. 24e). The kinetic limitation intrinsic to single-crystal particles can be alleviated through synergistic interactions with polycrystal or small-crystal materials. This strategy reduces the Li+ concentration gradient and causes the strain inside particles to benefit from the structural stability of single-crystal oxides during cycling.
Inspired by the morphology of LiCoO2, the strategy of nickel-based layered oxides has gradually transformed from atom substitution and surface modification to morphological design and then to low-cobalt nickel-rich cathodes with a high capacity density, low cost, and improved safety. Figure 25 shows the development history of single-crystal layered oxides over a long period of time. The varieties of single-crystal forms are synthesized by using different methods, which are also summarized in Table 1.
Despite single-crystal nickel-rich layered oxides having been closely investigated, they still suffer from problems such as interfacial defects [186], fatigue degradation, and kinetic limitations. It is well accepted that single-crystal materials are theoretically superior to their polycrystal counterparts. Many modifications have been applied to single-crystal oxides to further improve their performance, yet most of the research on this aspect has not been made outstanding progress. Perhaps there are more efforts needed to design single-crystal oxides, such as new synthesis methods, particle sizes, and surface protections. Comprehensive thermal stability testing of single-crystal materials and a lateral comparison among single-crystal materials with different nickel contents are still absent in mechanics studies.
8 Concluding Remarks and Perspectives
The intensification of research interest in single-crystal layered oxides in recent years indicates that single-crystal technology holds promise for use in next-generation LIBs. Considering their cycling capability and thermal stability, single-crystal oxides have contributed to replacing polycrystal oxides to help LIBs achieve the goals of their next stage. Many critical findings on single-crystal oxide cathodes have been reported in some well-known journals [181,182,183,184,185]. This article promptly reviews related works from scientists to further promote the development of single-crystal oxide cathodes. In this context, the following perspectives for single-crystal technology are considered.
-
I.
Development direction. Nickel is of great interest for the production of layered oxide cathodes because of its lower cost, wider availability, and lower toxicity than other constituent elements. With increasing nickel content, layered oxides can deliver increased energy in LIBs but decreased thermal stability and cyclability. Doping and coating are efficient strategies for delaying the onset of damage but fail to overcome degradation and are unsuitable for industrial production. Considering the good qualities of single-crystal materials, developing a path to single-crystal cathodes for layered oxides with ultrahigh nickel content is absolutely imperative.
-
II.
Synthesis problems. Two broad classes of synthesis processes are presented in the majority of the literature: molten salt and high-temperature methods. In the molten salt method, various oxides with low eutectic points are utilized to promote micron-scale grain growth at low temperature. This method is dominated by the dissolution–recrystallization mechanism. However, it is plagued by the problem of finding the appropriate molten salt to prevent the introduction of extrinsic components into the layered structure that cause the low energy density per kg shown by cathode materials. The high-temperature strategy is applied to layered oxides with low and moderate nickel contents [187]. Nickel-rich oxides suffer from structural deficiency in their interiors and on their surfaces as a result of lithium/oxygen loss at higher temperature, which is attributed to the sensitivity of nickel to temperature. Recent works on single-crystal materials have adopted the molten salt method instead of the high-temperature method and have included comparative studies on different salts. As reported by researchers, the preferred growth of crystal facets can improve the transport of lithium ions in the presence of any kind of salt.
-
III.
Follow-up technologies. Additional processing procedures, including grinding, sieving, and washing, have a considerable effect on the electrochemical performance of single-crystal materials. The high-temperature and molten salt methods are prone to inducing the agglomeration of layered oxides because of their growth mechanism. Grinding and sieving are two important steps for producing single-crystal materials with well-distributed sizes. Regarding grinding, the mechanical properties of layered oxides with different nickel contents should be considered. Furthermore, in commercial cathodes, a bimodal distribution of large and small particles is applied to increase the power tap/pellet density. Single-crystal materials can exploit the advantage of tuning particle sizes to maximize volumetric energy density. However, large amounts of residual salt and excess lithium are present at high temperature because of the molten salt method, which must be eradicated from the material by washing. Thus, an appropriate molten salt must be developed to not only effectively help grain growth but also facilitate the removal of the salt.
-
IV.
Electrochemical properties. Grain sizes vary in accordance with the different synthesis technologies, and their effect on the specific capacity, kinetics limitations (lithium diffusion and charge transfer), and thermal stability remains unclear. Many efforts are needed to explore the relationship between the properties (e.g., crystal structure, electronic structure, and morphology) and performance of single-crystal materials to provide insights into the rational design of cathode materials with thermal and cycling stability. Compared with polycrystal cathodes, research on single-crystal cathodes, especially on their modification strategies, is still at its fledgling stage. The theoretical calculation and simulation modeling techniques for the analysis of polycrystal materials are equally applicable for the analysis of single-crystal materials. Finally, a stable interfacial design contributes to the good performance of single-crystal materials with high specific surface area ratios.
References
Mizushima, K., Jones, P.C., Wiseman, P.J., et al.: LixCoO2 (0 < x < − 1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980). https://doi.org/10.1016/0025-5408(80)90012-4
Li, H.Y., Zhang, N., Li, J., et al.: Updating the structure and electrochemistry of LixNiO2 for 0 \(\leqslant\) x \(\leqslant\) 1. J. Electrochem. Soc. 165, A2985–A2993 (2018). https://doi.org/10.1149/2.0381813jes
Li, W., Asl, H.Y., Xie, Q., et al.: Collapse of LiNi1–x–yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019). https://doi.org/10.1021/jacs.8b13798
Wang, L., Wu, B.R., Mu, D.B., et al.: Single-crystal LiNi0.6Co0.2Mn0.2O2 as high performance cathode materials for Li-ion batteries. J. Alloy. Compd. 674, 360–367 (2016). https://doi.org/10.1016/j.jallcom.2016.03.061
Tarascon, J.M., Armand, M.: Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). https://doi.org/10.1038/35104644
Ohzuku, T., Ueda, A., Nagayama, M., et al.: Comparative study of LiCoO2, LiNi12Co12O2 and LiNiO2 for 4 volt secondary lithium cells. Electrochim Acta 38, 1159–1167 (1993). https://doi.org/10.1016/0013-4686(93)80046-3
Chen, C.H., Liu, J., Stoll, M.E., et al.: Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sour. 128, 278–285 (2004). https://doi.org/10.1016/j.jpowsour.2003.10.009
Kalyani, P., Kalaiselvi, N.: Various aspects of LiNiO2 chemistry: a review. Sci. Technol. Adv. Mater. 6, 689–703 (2005). https://doi.org/10.1016/j.stam.2005.06.001
Kitada, K., Murayama, H., Fukuda, K., et al.: Factors determining the packing-limitation of active materials in the composite electrode of lithium-ion batteries. J. Power Sour. 301, 11–17 (2016). https://doi.org/10.1016/j.jpowsour.2015.09.105
Zhang, L.W.: The push to high nickel content in layered oxides-production, cost, and supply & demand (in Chinese). Securities Research Report of Shanxi Securities Co., LTD. (2019). Accessed 13 September 2021. http://www.nxny.com/Report/View_4047585.html
Bianchini, M., Roca-Ayats, M., Hartmann, P., et al.: There and back again: the journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 58, 10434–10458 (2019). https://doi.org/10.1002/anie.201812472
Li, W.D., Erickson, E.M., Manthiram, A.: High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020). https://doi.org/10.1038/s41560-019-0513-0
Li, W.D., Song, B.H., Manthiram, A.: High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017). https://doi.org/10.1039/C6CS00875E
Myung, S.T., Maglia, F., Park, K.J., et al.: Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett. 2, 196–223 (2017). https://doi.org/10.1021/acsenergylett.6b00594
Sun, H.H., Ryu, H.H., Kim, U.H., et al.: Beyond doping and coating: prospective strategies for stable high-capacity layered Ni-rich cathodes. ACS Energy Lett. 5, 1136–1146 (2020). https://doi.org/10.1021/acsenergylett.0c00191
Wang, X.X., Ding, Y.L., Deng, Y.P., et al.: Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: promises and challenges. Adv. Energy Mater. 10, 1903864 (2020). https://doi.org/10.1002/aenm.201903864
Whittingham, M.S.: Special editorial perspective: beyond Li-ion battery chemistry. Chem. Rev. 120, 6328–6330 (2020). https://doi.org/10.1021/acs.chemrev.0c00438
Zhang, S.S.: Challenges and strategies for fast charge of Li-ion batteries. ChemElectroChem 7, 3569–3577 (2020). https://doi.org/10.1002/celc.202000650
Zhao, W.J.: A forum on batteries: from lithium-ion to the next generation. Natl. Sci. Rev. 7, 1263–1268 (2020). https://doi.org/10.1093/nsr/nwaa068
Su, Z., Liu, J.H., Li, M., et al.: Defect engineering in titanium-based oxides for electrochemical energy storage devices. Electrochem. Energy Rev. 3, 286–343 (2020). https://doi.org/10.1007/s41918-020-00064-5
Urban, A., Abdellahi, A., Dacek, S., et al.: Electronic-structure origin of cation disorder in transition-metal oxides. Phys. Rev. Lett. 119, 176402 (2017). https://doi.org/10.1103/physrevlett.119.176402
Li, J., Zhang, N., Li, H.Y., et al.: Impact of the synthesis conditions on the performance of LiNixCoyAlzO2 with high Ni and low Co content. J. Electrochem. Soc. 165, A3544–A3557 (2018). https://doi.org/10.1149/2.0931814jes
Kim, J., Lee, H., Cha, H., et al.: Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 8, 1702028 (2018). https://doi.org/10.1002/aenm.201702028
Huang, Y.C., Yang, H., Xiong, T.Z., et al.: Adsorption energy engineering of nickel oxide hybrid nanosheets for high areal capacity flexible lithium-ion batteries. Energy Storage Mater. 25, 41–51 (2020). https://doi.org/10.1016/j.ensm.2019.11.001
Li, W.D., Liu, X.M., Celio, H., et al.: Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability. Adv. Energy Mater. 8, 1703154 (2018). https://doi.org/10.1002/aenm.201703154
Radin, M.D., Hy, S., Sina, M., et al.: Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 7, 1602888 (2017). https://doi.org/10.1002/aenm.201602888
MacNeil, D.D., Lu, Z., Dahn, J.R.: Structure and electrochemistry of Li[NixCo1−2xMnx]O2 (0 \(\leqslant\) x \(\leqslant\) 1/2). J. Electrochem. Soc. 148, A1332–A1336 (2002). https://doi.org/10.1149/1.1505633
Hwang, B.J., Tsai, Y.W., Carlier, D., et al.: A combined computational/experimental study on LiNi1/3Co1/3Mn1/3O2 Chem. Mater. 15, 3676–3682 (2003). https://doi.org/10.1021/cm030299v
Lin, F., Liu, Y.J., Yu, X.Q., et al.: Synchrotron X-ray analytical techniques for studying materials electrochemistry in rechargeable batteries. Chem. Rev. 117, 13123–13186 (2017). https://doi.org/10.1021/acs.chemrev.7b00007
Liu, J.X., Wang, J.Q., Ni, Y.X., et al.: Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 43, 132–165 (2021). https://doi.org/10.1016/j.mattod.2020.10.028
Delmas, C., Ménétrier, M., Croguennec, L., et al.: An overview of the Li(Ni, M)O2 systems: syntheses, structures and properties. Electrochim. Acta 45, 243–253 (1999). https://doi.org/10.1016/S0013-4686(99)00208-X
Jang, Y.I., Huang, B.Y., Wang, H.F., et al.: Synthesis and characterization of LiAlyCo1−yO2 and LiAlyNi1−yO2. J. Power Sour. 81/82, 589–593 (1999). https://doi.org/10.1016/S0378-7753(99)00104-4
Koyama, Y., Tanaka, I., Adachi, H., et al.: Crystal and electronic structures of superstructural Li1−x[Co1/3Ni1/3Mn1/3]O2 (0 \(\leqslant\) x \(\leqslant\) 1). J. Power Sour. 119(120/121), 644–648 (2003). https://doi.org/10.1016/S0378-7753(03)00194-0
Kim, U.H., Kim, J.H., Hwang, J.Y., et al.: Compositionally and structurally redesigned high-energy Ni-rich layered cathode for next-generation lithium batteries. Mater. Today 23, 26–36 (2019). https://doi.org/10.1016/j.mattod.2018.12.004
Lee, S.Y., Park, G.S., Jung, C., et al.: Revisiting primary particles in layered lithium transition-metal oxides and their impact on structural degradation. Adv. Sci. 6, 1800843 (2019). https://doi.org/10.1002/advs.201800843
Van Bommel, A., Dahn, J.R.: Analysis of the growth mechanism of coprecipitated spherical and dense nickel, manganese, and cobalt-containing hydroxides in the presence of aqueous ammonia. Chem. Mater. 21, 1500–1503 (2009). https://doi.org/10.1021/cm803144d
Qiu, H.F., Wang, Y.L., Ye, S.H.: Rationally-directed synthesis and characterization of nickel-rich cathode material for lithium ion battery. Energy Technol. 6, 2419–2428 (2018). https://doi.org/10.1002/ente.201800415
Bianchini, M., Fauth, F., Hartmann, P., et al.: An in situ structural study on the synthesis and decomposition of LiNiO2. J. Mater. Chem. A 8, 1808–1820 (2020). https://doi.org/10.1039/c9ta12073d
Wang, C.H., Shao, L., Guo, X., et al.: Air-induced degradation and electrochemical regeneration for the performance of layered Ni-rich cathodes. ACS Appl. Mater. Inter. 11, 44036–44045 (2019). https://doi.org/10.1021/acsami.9b11452
Ceder, G., Van der Ven, A.: Phase diagrams of lithium transition metal oxides: investigations from first principles. Electrochim. Acta 45, 131–150 (1999). https://doi.org/10.1016/S0013-4686(99)00199-1
Wolverton, C., Zunger, A.: Prediction of Li intercalation and battery voltages in layered vs. cubic LixCoO2. J. Electrochem. Soc. 145, 2424–2431 (1998). https://doi.org/10.1149/1.1838653
Rahman, M.K., Saito, Y.: Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells: III: an approach to the power fade mechanism using FT-IR-ATR. J. Power Sour. 174, 889–894 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.222
Jung, S.K., Gwon, H., Hong, J., et al.: Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 4, 1300787 (2014). https://doi.org/10.1002/aenm.201300787
Hwang, S., Chang, W., Kim, S.M., et al.: Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem. Mater. 26, 1084–1092 (2014). https://doi.org/10.1021/cm403332s
Streich, D., Erk, C., Guéguen, A., et al.: Operando monitoring of early Ni-mediated surface reconstruction in layered lithiated Ni–Co–Mn oxides. J. Phys. Chem. C 121, 13481–13486 (2017). https://doi.org/10.1021/acs.jpcc.7b02303
Flores, E., Vonrüti, N., Novák, P., et al.: Elucidation of LixNi0.8Co0.15Al0.05O2 redox chemistry by operando raman spectroscopy. Chem. Mater 30, 4694–4703 (2018). https://doi.org/10.1021/acs.chemmater.8b01384
Jung, R., Metzger, M., Maglia, F., et al.: Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 164, A1361–A1377 (2017). https://doi.org/10.1149/2.0021707jes
Kong, F.T., Liang, C.P., Wang, L.H., et al.: Kinetic stability of bulk LiNiO2 and surface degradation by oxygen evolution in LiNiO2-based cathode materials. Adv. Energy Mater. 9, 1802586 (2019). https://doi.org/10.1002/aenm.201802586
Hatsukade, T., Schiele, A., Hartmann, P., et al.: Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes. ACS Appl. Mater. Interf. 10, 38892–38899 (2018). https://doi.org/10.1021/acsami.8b13158
Zhang, S.S.: Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials. J. Energy Chem. 41, 135–141 (2020). https://doi.org/10.1016/j.jechem.2019.05.013
House, R.A., Maitra, U., Jin, L.Y., et al.: What triggers oxygen loss in oxygen redox cathode materials? Chem. Mater. 31, 3293–3300 (2019). https://doi.org/10.1021/acs.chemmater.9b00227
Lin, F., Markus, I.M., Nordlund, D., et al.: Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014). https://doi.org/10.1038/ncomms4529
Cherkashinin, G., Motzko, M., Schulz, N., et al.: Electron spectroscopy study of Li[Ni, Co, Mn]O2/electrolyte interface: electronic structure, interface composition, and device implications. Chem. Mater. 27, 2875–2887 (2015). https://doi.org/10.1021/cm5047534
Li, J.Y., Manthiram, A.: A comprehensive analysis of the interphasial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries. Adv. Energy Mater. 9, 1902731 (2019). https://doi.org/10.1002/aenm.201902731
Zheng, S.J., Huang, R., Makimura, Y., et al.: Microstructural changes in LiNi0.8Co0.15Al0.05O2 positive electrode material during the first cycle. J. Electrochem. Soc. 158, A357–A362 (2011). https://doi.org/10.1149/13544843
Busche, M.R., Drossel, T., Leichtweiss, T., et al.: Dynamic formation of a solid-liquid electrolyte interphase and its consequences for hybrid-battery concepts. Nat. Chem. 8, 426–434 (2016). https://doi.org/10.1038/nchem.2470
Gilbert, J.A., Bareño, J., Spila, T., et al.: Cycling behavior of NCM523/graphite lithium-ion cells in the 3–4.4V range: diagnostic studies of full cells and harvested electrodes. J. Electrochem. Soc. 164, A6054–A6065 (2016). https://doi.org/10.1149/2.0081701jes
Zheng, H.H., Sun, Q.N., Liu, G., et al.: Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sour. 207, 134–140 (2012). https://doi.org/10.1016/j.jpowsour.2012.01.122
Morin, H.R., Graczyk, D.G., Tsai, Y., et al.: Transition-metal dissolution from NMC-family oxides: a case study. ACS Appl. Energy Mater. 3, 2565–2575 (2020). https://doi.org/10.1021/acsaem.9b02277
Sahore, R., O Hanlon, D.C., Tornheim, A., et al.: Revisiting the mechanism behind transition-metal dissolution from delithiated LiNixMnyCozO2 (NMC) cathodes. J. Electrochem. Soc. 167, 020513 (2020). https://doi.org/10.1149/1945-7111/ab6826
Li, W., Dolocan, A., Oh, P., et al.: Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries. Nat. Commun. 8, 14589 (2017). https://doi.org/10.1038/ncomms14589
Gent, W.E., Li, Y.Y., Ahn, S., et al.: Persistent state-of-charge heterogeneity in relaxed, partially charged Li1−xNi1/3Co1/3Mn1/3O2 secondary particles. Adv. Mater. 28, 6631–6638 (2016). https://doi.org/10.1002/adma.201601273
Mao, Y.W., Wang, X.L., Xia, S.H., et al.: High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 29, 1900247 (2019). https://doi.org/10.1002/adfm.201900247
Xu, Z.R., Jiang, Z.S., Kuai, C.G., et al.: Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 11, 83 (2020). https://doi.org/10.1038/s41467-019-13884-x
Liu, P.F., Xu, R., Liu, Y.J., et al.: Computational modeling of heterogeneity of stress, charge, and cyclic damage in composite electrodes of Li-ion batteries. J. Electrochem. Soc. 167, 040527 (2020). https://doi.org/10.1149/1945-7111/ab78fa
Li, H.Y., Liu, A., Zhang, N., et al.: An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries. Chem. Mater. 31, 7574–7583 (2019). https://doi.org/10.1021/acs.chemmater.9b02372
Yan, P., Zheng, J., Gu, M., et al.: Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat Commun. 8, 14101 (2017). https://doi.org/10.1038/ncomms14101
Kim, J.H., Ryu, H.H., Kim, S.J., et al.: Degradation mechanism of highly Ni-rich Li[NixCoyMn1–x–y]O2 cathodes with x > 09. ACS Appl. Mater. Interf. 11, 30936–30942 (2019). https://doi.org/10.1021/acsami.9b09754
Ruess, R., Schweidler, S., Hemmelmann, H., et al.: Influence of NCM particle cracking on kinetics of lithium-ion batteries with liquid or solid electrolyte. J. Electrochem. Soc. 167, 100532 (2020). https://doi.org/10.1149/1945-7111/ab9a2c
Yang, Y., Xu, R., Zhang, K., et al.: Quantification of heterogeneous degradation in Li-ion batteries. Adv. Energy Mater. 9, 1900674 (2019). https://doi.org/10.1002/aenm.201900674
Bai, Y., Zhao, K.J., Liu, Y., et al.: A chemo-mechanical grain boundary model and its application to understand the damage of Li-ion battery materials. Scr. Mater. 183, 45–49 (2020). https://doi.org/10.1016/j.scriptamat.2020.03.027
Mao, C.Y., Ruther, R.E., Geng, L.X., et al.: Evaluation of gas formation and consumption driven by crossover effect in high-voltage lithium-ion batteries with Ni-rich NMC cathodes. ACS Appl. Mater. Interf. 11, 43235–43243 (2019). https://doi.org/10.1021/acsami.9b15916
Palacin, M.R., de Guibert, A.: Why do batteries fail? Science 351, 1253292 (2016). https://doi.org/10.1126/science.1253292
Kong, D.F., Zhang, M.J., Xiao, Y.G., et al.: Insights into the structural evolution and Li/O loss in high-Ni layered oxide cathodes. Nano Energy 59, 327–335 (2019). https://doi.org/10.1016/j.nanoen.2019.02.059
Yan, P.F., Zheng, J.M., Chen, T.W., et al.: Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat. Commun. 9, 2437 (2018). https://doi.org/10.1038/s41467-018-04862-w
Yang, H., Zhuang, G.V., Ross, P.N., Jr.: Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sour. 161, 573–579 (2006). https://doi.org/10.1016/j.jpowsour.2006.03.058
Noh, H.J., Youn, S., Yoon, C.S., et al.: Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sour. 233, 121–130 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.063
Liu, X., Ren, D.S., Hsu, H., et al.: Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2, 2047–2064 (2018). https://doi.org/10.1016/j.joule.2018.06.015
Ryu, H.H., Park, K.J., Yoon, C.S., et al.: Capacity fading of Ni-rich Li[NixCoyMn1–x–y]O2 (0.6 \(\leqslant\) x \(\leqslant\) 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation? Chem. Mater. 30, 1155–1163 (2018). https://doi.org/10.1021/acs.chemmater.7b05269
Lim, J.M., Hwang, T., Kim, D., et al.: Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 7, 39669 (2017). https://doi.org/10.1038/srep39669
Nam, G.W., Park, N.Y., Park, K.J., et al.: Capacity fading of Ni-rich NCA cathodes: effect of microcracking extent. ACS Energy Lett. 4, 2995–3001 (2019). https://doi.org/10.1021/acsenergylett.9b02302
Hackley, V. A., Ferraris, C.F.: The use of nomenclature in dispersion science and technology. U.S. Department of Commerce, Technology Administration, National Institute of Standards and Technology, Washington (2001)
Kim, U.H., Ryu, H.H., Kim, J.H., et al.: Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles. Adv. Energy Mater. 9, 1803902 (2019). https://doi.org/10.1002/aenm.201803902
Sun, Y.K., Myung, S.T., Park, B.C., et al.: High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 8, 320–324 (2009). https://doi.org/10.1038/nmat2418
Sun, H.H., Weeks, J.A., Heller, A., et al.: Nanorod gradient cathode: preventing electrolyte penetration into cathode particles. ACS Appl. Energy Mater. 2, 6002–6011 (2019). https://doi.org/10.1021/acsaem.9b01116
Xu, X., Xiang, L.Z., Wang, L.G., et al.: Progressive concentration gradient nickel-rich oxide cathode material for high-energy and long-life lithium-ion batteries. J. Mater. Chem. A 7, 7728–7735 (2019). https://doi.org/10.1039/c9ta00224c
Zhang, N., Zaker, N., Li, H.Y., et al.: Cobalt-free nickel-rich positive electrode materials with a core–shell structure. Chem. Mater. 31, 10150–10160 (2019). https://doi.org/10.1021/acs.chemmater.9b03515
Sun, Y.K., Chen, Z., Noh, H.J., et al.: Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 11, 942–947 (2012). https://doi.org/10.1038/nmat3435
Ryu, H.H., Park, N.Y., Yoon, D.R., et al.: New class of Ni-rich cathode materials Li[NixCoyB1–x–y]O2 for next lithium batteries. Adv. Energy Mater. 10, 2000495 (2020). https://doi.org/10.1002/aenm.202000495
Yang, X., Tang, Y.W., Shang, G.Z., et al.: Enhanced cyclability and high-rate capability of LiNi0.88Co0.095Mn0.025O2 cathodes by homogeneous Al3+ doping. ACS Appl. Mater. Interf. 11, 32015–32024 (2019). https://doi.org/10.1021/acsami.9b10558
Ryu, H.H., Park, K.J., Yoon, D.R., et al.: Cathodes: Li[Ni0.9Co0.09W0.01]O2: a new type of layered oxide cathode with high cycling stability. Adv. Energy Mater. 9, 1970174 (2019). https://doi.org/10.1002/aenm.201970174
Xu, X., Huo, H., Jian, J.Y., et al.: Lithium-ion batteries: radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8 Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 9, 1970051 (2019). https://doi.org/10.1002/aenm.201970051
Jiang, J., Dahn, J.R.: Effects of particle size and electrolyte salt on the thermal stability of Li0.5CoO2. Electrochim Acta 49, 2661–2666 (2004). https://doi.org/10.1016/j.electacta.2004.02.017
Li, D.S., Nielsen, M.H., Lee, J.R.I., et al.: Direction-specific interactions control crystal growth by oriented attachment. Science 336, 1014–1018 (2012). https://doi.org/10.1126/science.1219643
Zhao, J.Q., Zhang, W., Huq, A., et al.: In situ probing and synthetic control of cationic ordering in Ni-rich layered oxide cathodes. Adv. Energy Mater. 7, 1601266 (2017). https://doi.org/10.1002/aenm.201601266
Bao, W., Qian, G., Zhao, L., et al.: Simultaneous enhancement of interfacial stability and kinetics of single-crystal LiNi0.6Mn0.2Co0.2O2 through optimized surface coating and doping. Nano Lett. 20, 8832–8840 (2020). https://doi.org/10.1021/acs.nanolett.0c03778
Kim, Y.: Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: morphology and performance as a cathode material for lithium ion batteries. ACS Appl. Mater. Interf. 4, 2329–2333 (2012). https://doi.org/10.1021/am300386j
Bugaris, D.E., Zur Loye, H.C.: Materials discovery by flux crystal growth: quaternary and higher order oxides. Angew. Chem. Int. Ed. 51, 3780–3811 (2012). https://doi.org/10.1002/anie.201102676
Zhu, J., Chen, G.Y.: Single-crystal based studies for correlating the properties and high-voltage performance of Li[NixMnyCo1–x–y]O2 cathodes. J. Mater. Chem. A 7, 5463–5474 (2019). https://doi.org/10.1039/c8ta10329a
Kimijima, T., Zettsu, N., Yubuta, K., et al.: Molybdate flux growth of idiomorphic Li(Ni1/3Co1/3Mn1/3)O2 single crystals and characterization of their capabilities as cathode materials for lithium-ion batteries. J. Mater. Chem. A 4, 7289–7296 (2016). https://doi.org/10.1039/c6ta01593j
Chang, Z.R., Yu, X., Tang, H.W., et al.: Synthesis of LiNi1/3Co1/3Al1/3O2 cathode material with eutectic molten salt LiOH–LiNO3. Powder Tech. 207, 396–400 (2011). https://doi.org/10.1016/j.powtec.2010.11.025
Spence, S.L., Xu, Z.R., Sainio, S., et al.: Tuning the morphology and electronic properties of single-crystal LiNi0.5Mn1.5O4–δ: exploring the influence of LiCl–KCl molten salt flux composition and synthesis temperature. Inorg. Chem. 59, 10591–10603 (2020). https://doi.org/10.1021/acs.inorgchem.0c01042
Liu, X.L., Wu, J.J., Huang, X.L., et al.: Predominant growth orientation of Li1.2(Mn0.4Co0.4)O2 cathode materials produced by the NaOH compound molten salt method and their enhanced electrochemical performance. J. Mater. Chem. A 2, 15200 (2014). https://doi.org/10.1039/c4ta02841d
Liang, R., Wu, Z.Y., Yang, W.M., et al.: A simple one-step molten salt method for synthesis of micron-sized single primary particle LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Ionics 26, 1635–1643 (2020). https://doi.org/10.1007/s11581-020-03500-0
Huang, B., Wang, M., Zhang, X.W., et al.: Synergistic coupling effect of single crystal morphology and precursor treatment of Ni-Rich cathode materials. J. Alloy. Compd. 830, 154619 (2020). https://doi.org/10.1016/j.jallcom.2020.154619
Qian, G.N., Zhang, Y.T., Li, L.S., et al.: Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 27, 140–149 (2020). https://doi.org/10.1016/j.ensm.2020.01.027
Kimijima, T., Zettsu, N., Teshima, K.: Growth manner of octahedral-shaped Li(Ni1/3Co1/3Mn1/3)O2 single crystals in molten Na2SO4. Cryst. Growth Des. 16, 2618–2623 (2016). https://doi.org/10.1021/acs.cgd.5b01723
Qu, Y.Y., Mo, Y., Jia, X.B., et al.: Flux growth and enhanced electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode material by excess lithium carbonate for lithium-ion batteries. J. Alloy. Compd. 788, 810–818 (2019). https://doi.org/10.1016/j.jallcom.2019.02.285
Wang, T., Ren, K.L., He, M., et al.: Synthesis and manipulation of single-crystalline lithium nickel manganese cobalt oxide cathodes: a review of growth mechanism. Front. Chem. 8, 747 (2020). https://doi.org/10.3389/fchem.2020.00747
Juillerat, C.A., Klepov, V.V., Morrison, G., et al.: Flux crystal growth: a versatile technique to reveal the crystal chemistry of complex uranium oxides. Dalton Trans. Camb. Engl. 48, 3161–3181 (2019). https://doi.org/10.1039/c8dt04675a
Jung, R., Morasch, R., Karayaylali, P., et al.: Effect of ambient storage on the degradation of Ni-rich positive electrode materials (NMC811) for Li-ion batteries. J. Electrochem. Soc. 165, A132–A141 (2018). https://doi.org/10.1149/2.0401802jes
Sicklinger, J., Metzger, M., Beyer, H., et al.: Ambient storage derived surface contamination of NCM811 and NCM111: performance implications and mitigation strategies. J. Electrochem. Soc. 166, A2322–A2335 (2019). https://doi.org/10.1149/2.0011912jes
Faenza, N.V., Bruce, L., Lebens-Higgins, Z.W., et al.: Editors’ choice—growth of ambient induced surface impurity species on layered positive electrode materials and impact on electrochemical performance. J. Electrochem. Soc. 164, A3727–A3741 (2017). https://doi.org/10.1149/2.0921714jes
Larcher, D., Palacín, M.R., Amatucci, G.G., et al.: Electrochemically active LiCoO2 and LiNiO2 made by cationic exchange under hydrothermal conditions. J. Electrochem. Soc. 144, 408–417 (1997). https://doi.org/10.1149/1.1837424
Lin, C.H., Zhang, Y.Z., Chen, L., et al.: Hydrogen peroxide assisted synthesis of LiNi1/3Co1/3Mn1/3O2 as high-performance cathode for lithium-ion batteries. J. Power Sour. 280, 263–271 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.084
Wang, H., Wei, Y.J., Wang, J.T., et al.: Polymer-chelation synthesis of compositionally homogeneous LiNi1/3Co1/3Mn1/3O2 crystals for lithium-ion cathode. Electrochim. Acta 269, 724–732 (2018). https://doi.org/10.1016/j.electacta.2018.03.029
Huang, D., Shi, Y., Tornheim, A.P., et al.: Nanoscale LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries via a polymer-assisted chemical solution method. Appl. Mater. Today 16, 342–350 (2019). https://doi.org/10.1016/j.apmt.2019.06.008
Wu, F., Wang, M., Su, Y.F., et al.: A novel layered material of LiNi0.32Mn0.33Co0.33Al0.01O2 for advanced lithium-ion batteries. J. Power Sour. 195, 2900–2904 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.041
Huang, Z.D., Liu, X.M., Oh, S.W., et al.: Microscopically porous, interconnected single crystal LiNi1/3Co1/3Mn1/3O2 cathode material for Lithium ion batteries. J. Mater. Chem. 21, 10777–10784 (2011). https://doi.org/10.1039/c1jm00059d
Fu, F., Xu, G.L., Wang, Q., et al.: Synthesis of single crystalline hexagonal nanobricks of LiNi1/3Co1/3Mn1/3O2 with high percentage of exposed 010 active facets as high rate performance cathode material for lithium-ion battery. J. Mater. Chem. A 1, 3860–3864 (2013). https://doi.org/10.1039/c3ta01618h
Ma, Z., Yu, J.H., Dai, S.: Preparation of inorganic materials using ionic liquids. Adv. Mater. 22, 261–285 (2010). https://doi.org/10.1002/adma.200900603
Kobayashi, S., Usui, T., Ikuta, H., et al.: Preparation of cathode active materials for lithium ion secondary batteries utilizing microwave irradiation: Part II. Electronic structure of lithium manganese oxide. J. Mater. Res. 19, 2421–2427 (2004). https://doi.org/10.1557/JMR.2004.0313
Zhu, J., Zheng, J.C., Cao, G.L., et al.: Flux-free synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 boosts its electrochemical performance in lithium batteries. J. Power Sour. 464, 228207 (2020). https://doi.org/10.1016/j.jpowsour.2020.228207
Lu, Y., Gan, Z.G., Xia, J., et al.: Hydrothermal synthesis of tunable olive-like Ni0.8Co0.1Mn0.1CO3 and its transformation to LiNi0.8Co0.1Mn0.1O2 cathode materials for Li-ion batteries. ChemElectroChem 6, 5661–5670 (2019). https://doi.org/10.1002/celc.201901539
Zhang, L.S., Wang, H., Wang, L.Z., et al.: High electrochemical performance of hollow corn-like LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Appl. Surf. Sci. 450, 461–467 (2018). https://doi.org/10.1016/j.apsusc.2018.04.224
Park, K., Park, J.H., Hong, S.G., et al.: Enhancement in the electrochemical performance of zirconium/phosphate bi-functional coatings on LiNi0.8Co0.15Mn0.05O2 by the removal of Li residuals. Phys. Chem. Chem. Phys. 18, 29076–29085 (2016). https://doi.org/10.1039/c6cp05286j
Cheng, L., Zhang, B., Su, S.L., et al.: Comparison of monocrystalline and secondary LiNi0.5Co0.2Mn0.3O2 cathode material for high-performance lithium-ion batteries. J. Alloy. Compd. 845, 156202 (2020). https://doi.org/10.1016/j.jallcom.2020.156202
Li, J., Li, H.Y., Stone, W., et al.: Synthesis of single crystal LiNi0.5Mn0.3Co0.2O2for lithium ion batteries. J. Electrochem. Soc. 164, A3529–A3537 (2017). https://doi.org/10.1149/2.0401714jes
Li, H.Y., Li, J., Ma, X.W., et al.: Synthesis of single crystal LiNi0.6Mn0.2Co0.2O2with enhanced electrochemical performance for lithium ion batteries. J. Electrochem. Soc. 165, A1038–A1045 (2018). https://doi.org/10.1149/2.0951805jes
Li, H.Y., Li, J., Zaker, N., et al.: Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method. J. Electrochem. Soc. 166, A1956–A1963 (2019). https://doi.org/10.1149/2.0681910jes
Yan, W.W., Jia, X.B., Yang, S.Y., et al.: Synthesis of single crystal LiNi0.92Co0.06Mn0.01Al0.01O2 cathode materials with superior electrochemical performance for lithium ion batteries. J. Electrochem. Soc. 167, 120514 (2020). https://doi.org/10.1149/1945-7111/abacea
Jain, A., Mohan, A., Yusuf, S.M.: Optical floating-zone growth of single crystals of Li-ion battery material LiCoO2. J. Cryst. Growth 536, 125578 (2020). https://doi.org/10.1016/j.jcrysgro.2020.125578
Zhu, J.X., Vo, T., Li, D.S., et al.: Crystal growth of Li[Ni1/3Co1/3Mn1/3]O2 as a cathode material for high-performance lithium ion batteries. Cryst. Growth Des. 12, 1118–1123 (2012). https://doi.org/10.1021/cg200565n
Hu, Y.H., Zhao, X.H., Suo, Z.G.: Averting cracks caused by insertion reaction in lithium-ion batteries. J. Mater. Res. 25, 1007–1010 (2010). https://doi.org/10.1557/JMR.2010.0142
Fan, X.M., Hu, G.R., Zhang, B., et al.: Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 70, 104450 (2020). https://doi.org/10.1016/j.nanoen.2020.104450
Kim, J., Ma, H., Cha, H., et al.: A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy lithium-ion batteries. Energy Environ. Sci. 11, 1449–1459 (2018). https://doi.org/10.1039/c8ee00155c
Li, J., Cameron, A.R., Li, H.Y., et al.: Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 164, A1534–A1544 (2017). https://doi.org/10.1149/2.0991707jes
Liu, Y.Q., Zhang, Y., Wang, J.: Mesocrystals as a class of multifunctional materials. CrystEngComm 16, 5948–5967 (2014). https://doi.org/10.1039/c4ce00256c
Liu, G.L., Li, M.L., Wu, N.T., et al.: Single-crystalline particles: an effective way to ameliorate the intragranular cracking, thermal stability, and capacity fading of the LiNi0.6Co0.2Mn0.2O2 electrodes. J. Electrochem. Soc. 165, A3040–A3047 (2018). https://doi.org/10.1149/2.0491813jes
Weber, R., Fell, C.R., Dahn, J.R., et al.: Operando X-ray diffraction study of polycrystalline and single-crystal LixNi0.5Mn0.3Co0.2O2. J. Electrochem. Soc. 164, A2992–A2999 (2017). https://doi.org/10.1149/2.0441713jes
Li, J., Li, H.Y., Stone, W., et al.: Development of electrolytes for single crystal NMC532/artificial graphite cells with long lifetime. J. Electrochem. Soc. 165, A626–A635 (2018). https://doi.org/10.1149/2.0971803jes
Aiken, C.P., Xia, J., Wang, D.Y., et al.: An apparatus for the study of in situ gas evolution in Li-ion pouch cells. J. Electrochem. Soc. 161, A1548–A1554 (2014). https://doi.org/10.1149/2.0151410jes
Glazier, S.L., Petibon, R., Xia, J., et al.: Measuring the parasitic heat flow of lithium ion pouch cells containing EC-free electrolytes. J. Electrochem. Soc. 164, A567–A573 (2017). https://doi.org/10.1149/2.0331704jes
Liu, Y.L., Harlow, J., Dahn, J.: Microstructural observations of “single crystal” positive electrode materials before and after long term cycling by cross-section scanning electron microscopy. J. Electrochem. Soc. 167, 020512 (2020). https://doi.org/10.1149/1945-7111/ab6288
He, L.P., Li, K., Zhang, Y., et al.: Structural and electrochemical properties of low-cobalt-content LiNi0.6+xCo0.2–xMn0.2O2 (0.0 \(\leqslant\) x \(\leqslant\) 0.1) cathodes for lithium-ion batteries. ACS Appl. Mater. Interf. 12, 28253–28253 (2020). https://doi.org/10.1021/acsami.0c06824
Harlow, J.E., Ma, X.W., Li, J., et al.: A wide range of testing results on an excellent lithium-ion cell chemistry to be used as benchmarks for new battery technologies. J. Electrochem. Soc. 166, A3031–A3044 (2019). https://doi.org/10.1149/2.0981913jes
Chen, M., Zhao, E., Chen, D., et al.: Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 56, 8355–8362 (2017). https://doi.org/10.1021/acs.inorgchem.7b01035
Muto, S., Tatsumi, K., Kojima, Y., et al.: Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods. J. Power Sour. 205, 449–455 (2012). https://doi.org/10.1016/j.jpowsour.2012.01.071
Li, H., Zhou, P.F., Liu, F.M., et al.: Stabilizing nickel-rich layered oxide cathodes by magnesium doping for rechargeable lithium-ion batteries. Chem. Sci. 10, 1374–1379 (2019). https://doi.org/10.1039/c8sc03385d
Xie, Q., Li, W.D., Manthiram, A.: A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries. Chem. Mater. 31, 938–946 (2019). https://doi.org/10.1021/acs.chemmater.8b03900
Kondo, H., Takeuchi, Y., Sasaki, T., et al.: Effects of Mg-substitution in Li(Ni, Co, Al)O2 positive electrode materials on the crystal structure and battery performance. J. Power Sour. 174, 1131–1136 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.035
Li, Y.C., Xiang, W., Wu, Z.G., et al.: Construction of homogeneously Al3+ doped Ni rich Ni–Co–Mn cathode with high stable cycling performance and storage stability via scalable continuous precipitation. Electrochim. Acta 291, 84–94 (2018). https://doi.org/10.1016/j.electacta.2018.08.124
Woo, S.W., Myung, S.T., Bang, H., et al.: Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al, Mg) substitution Electrochim. Acta 54, 3851–3856 (2009). https://doi.org/10.1016/j.electacta.2009.01.048
Liu, Q., Su, X., Lei, D., et al.: Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 3, 936–943 (2018). https://doi.org/10.1038/s41560-018-0180-6
Kim, U.H., Myung, S.T., Yoon, C.S., et al.: Extending the battery life using an Al-doped Li[Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium ion batteries. ACS Energy Lett. 2, 1848–1854 (2017). https://doi.org/10.1021/acsenergylett.7b00613
Kim, U.H., Kuo, L.Y., Kaghazchi, P., et al.: Quaternary layered Ni-rich NCMA cathode for lithium-ion batteries. ACS Energy Lett. 4, 576–582 (2019). https://doi.org/10.1021/acsenergylett.8b02499
Zou, L.F., Li, J.Y., Liu, Z.Y., et al.: Lattice doping regulated interfacial reactions in cathode for enhanced cycling stability. Nat. Commun. 10, 3447 (2019). https://doi.org/10.1038/s41467-019-11299-2
Liu, D., Liu, S.Y., Zhang, C.C., et al.: Revealing the effect of Ti doping on significantly enhancing cyclic performance at a high cutoff voltage for Ni-rich LiNi0.8Co0.15Al0.05O2 cathode. ACS Sustain. Chem. Eng. 7, 10661–10669 (2019). https://doi.org/10.1021/acssuschemeng.9b01312
Sun, H.B., Cao, Z.L., Wang, T.R., et al.: Enabling high rate performance of Ni-rich layered oxide cathode by uniform titanium doping. Mater. Today Energy 13, 145–151 (2019). https://doi.org/10.1016/j.mtener.2019.05.003
Yoon, C.S., Kim, U.H., Park, G.T., et al.: Self-passivation of a LiNiO2 cathode for a lithium-ion battery through Zr doping. ACS Energy Lett. 3, 1634–1639 (2018). https://doi.org/10.1021/acsenergylett.8b00805
Susai, F.A., Kovacheva, D., Chakraborty, A., et al.: Improving performance of LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries by doping with molybdenum-ions: theoretical and experimental studies. ACS Appl. Energy Mater. 2, 4521–4534 (2019). https://doi.org/10.1021/acsaem.9b00767
Xin, F.X., Zhou, H., Chen, X.B., et al.: Li–Nb–O coating/substitution enhances the electrochemical performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) cathode. ACS Appl. Mater. Interf. 11, 34889–34894 (2019). https://doi.org/10.1021/acsami.9b09696
Huang, Y., Liu, X., Yu, R.Z., et al.: Tellurium surface doping to enhance the structural stability and electrochemical performance of layered Ni-rich cathodes. ACS Appl. Mater. Interf. 11, 40022–40033 (2019). https://doi.org/10.1021/acsami.9b13906
Weigel, T., Schipper, F., Erickson, E.M., et al.: Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 4, 508–516 (2019). https://doi.org/10.1021/acsenergylett.8b02302
Chen, Y.P., Zhang, Y., Chen, B.J., et al.: An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sour. 256, 20–27 (2014). https://doi.org/10.1016/j.jpowsour.2014.01.061
Neudeck, S., Strauss, F., Garcia, G., et al.: Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun. 55, 2174–2177 (2019). https://doi.org/10.1039/c8cc09618j
Cho, W., Kim, S.M., Song, J.H., et al.: Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sour. 282, 45–50 (2015). https://doi.org/10.1016/j.jpowsour.2014.12.128
Lee, S.H., Yoon, C.S., Amine, K., et al.: Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating. J. Power Sour. 234, 201–207 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.045
Woo, S.U., Yoon, C.S., Amine, K., et al.: Significant improvement of electrochemical performance of AlF3-coated Li[Ni0.8Co0.1Mn0.1]O2 cathode materials. J. Electrochem. Soc. 154, A1005–A1009 (2007). https://doi.org/10.1149/1.2776160
Xie, J., Sendek, A.D., Cubuk, E.D., et al.: Atomic layer deposition of stable LiAlF4 lithium ion conductive interfacial layer for stable cathode cycling. ACS Nano 11, 7019–7027 (2017). https://doi.org/10.1021/acsnano.7b02561
Liu, K., Zhang, Q.Q., Dai, S., et al.: Synergistic effect of F– doping and LiF coating on improving the high-voltage cycling stability and rate capacity of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium-ion batteries. ACS Appl. Mater. Interf. 10, 34153–34162 (2018). https://doi.org/10.1021/acsami.8b10016
Jo, C.H., Cho, D.H., Noh, H.J., et al.: An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano. Res. 8, 1464–1479 (2015). https://doi.org/10.1007/s12274-014-0631-8
Lee, S.W., Kim, M.S., Jeong, J.H., et al.: Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: improved thermal stability and high-voltage performance. J. Power Sour. 360, 206–214 (2017). https://doi.org/10.1016/j.jpowsour.2017.05.042
Chen, Z., Kim, G.T., Guang, Y., et al.: Manganese phosphate coated Li[Ni0.6Co0.2Mn0.2]O2 cathode material: towards superior cycling stability at elevated temperature and high voltage. J. Power Sour. 402, 263–271 (2018). https://doi.org/10.1016/j.jpowsour.2018.09.049
Zeng, X.F., Zhu, J., Yang, L.S., et al.: Electrochemical stabilities of surface aluminum-doped LiNi0.5Co0.2Mn0.3O2 single crystals under different cutoff voltages. J. Electroanal. Chem. 838, 94–100 (2019). https://doi.org/10.1016/j.jelechem.2019.02.051
Zhao, Z.Y., Huang, B., Wang, M., et al.: Facile synthesis of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries. Solid State Ion. 342, 115065 (2019). https://doi.org/10.1016/j.ssi.2019.115065
Zeng, X.F., Jian, T.Z., Lu, Y., et al.: Enhancing high-temperature and high-voltage performances of single-crystal LiNi0.5Co0.2Mn0.3O2 cathodes through a LiBO2/LiAlO2 dual-modification strategy. ACS Sustain. Chem. Eng. 8, 6293–6304 (2020). https://doi.org/10.1021/acssuschemeng.9b07792
Zhang, M.L., Shen, J.T., Li, J., et al.: Effect of micron sized particle on the electrochemical properties of nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode materials. Ceram. Int. 46, 4643–4651 (2020). https://doi.org/10.1016/j.ceramint.2019.10.195
Zhu, J., Sharifi-Asl, S., Garcia, J.C., et al.: Atomic-level understanding of surface reconstruction based on Li[NixMnyCo1–x–y]O2 single-crystal studies. ACS Appl. Energy Mater. 3, 4799–4811 (2020). https://doi.org/10.1021/acsaem.0c00411
Gu, M., Belharouak, I., Genc, A., et al.: Conflicting roles of nickel in controlling cathode performance in lithium ion batteries. Nano Lett. 12, 5186–5191 (2012). https://doi.org/10.1021/nl302249v
Zhang, F., Lou, S.F., Li, S., et al.: Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 11, 3050 (2020). https://doi.org/10.1038/s41467-020-16824-2
Wang, C.H., Yu, R.Z., Hwang, S., et al.: Single crystal cathodes enabling high-performance all-solid-state lithium-ion batteries. Energy Stor. Mater. 30, 98–103 (2020). https://doi.org/10.1016/j.ensm.2020.05.007
Xu, C., Märker, K., Lee, J., et al.: Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2021). https://doi.org/10.1038/s41563-020-0767-8
Bi, Y., Tao, J., Wu, Y., et al.: Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020). https://doi.org/10.1126/science.abc3167
Ge, M.Y., Wi, S., Liu, X., et al.: Kinetic limitations in single-crystal high-nickel cathodes. Angew. Chem. Int. Ed. 60, 17350–17355 (2021). https://doi.org/10.1002/anie.202012773
Chen, H., Adekoya, D., Hencz, L., et al.: Stable seamless interfaces and rapid ionic conductivity of Ca–CeO2 /LiTFSI/PEO composite electrolyte for high-rate and high-voltage all-solid-state battery. Adv Energy Mater. 10, 2000049 (2020). https://doi.org/10.1002/aenm.202000049
Lu, S.J., Liu, Y., He, Z.J., et al.: Synthesis and properties of single-crystal Ni-rich cathode materials in Li-ion batteries. Trans. Nonferr. Met. Soc. China 31, 1074–1086 (2021). https://doi.org/10.1016/S1003-6326(21)65562-0
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51974368 and 51774333).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Sj., Tang, Lb., Wei, Hx. et al. Single-Crystal Nickel-Based Cathodes: Fundamentals and Recent Advances. Electrochem. Energy Rev. 5, 15 (2022). https://doi.org/10.1007/s41918-022-00166-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00166-2