This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nickel-laden black gold converts CO2 to chemicals using solar energy and green hydrogen
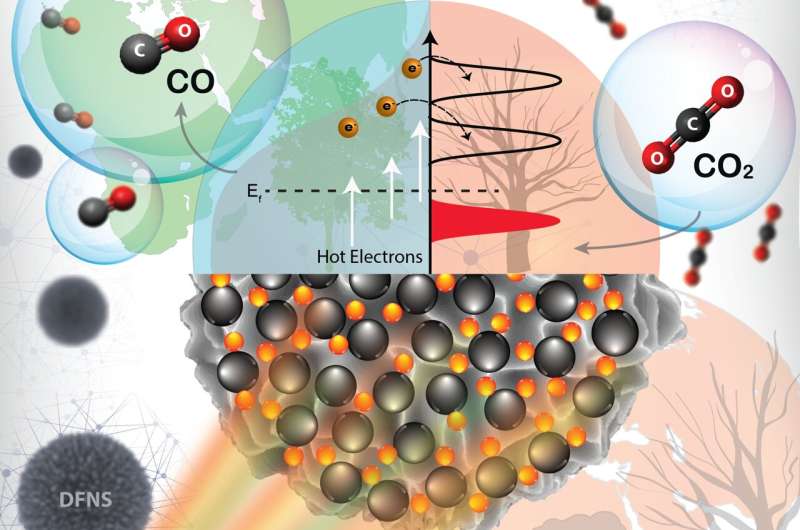
CO2 hydrogenation with green hydrogen is one of the best processes to combat climate change and can provide a single solution to three challenging problems, 1) excessive CO2 levels, 2) the temporal mismatch between solar electricity production and demand, and 3) hydrogen gas storage. However, the CO2 hydrogenation reaction needs very high temperatures, causing quick deactivation of the catalyst.
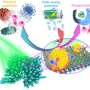
In new work published in ACS Nano, researchers at Tata Institute of Fundamental Research (TIFR), Mumbai, asked the question of whether this high-temperature CO2 hydrogenation can be catalyzed at room to moderate temperature via plasmonic excitation of H2 and CO2 using plasmonic catalyst. They have demonstrated that plasmonic black gold-nickel efficiently catalyzes CO2 hydrogenation using visible light.
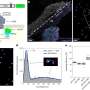
The reaction took place as low as 84 to 223°C without external heating. Researchers found a multifold increase in the catalytic activity as compared to DPC-C4 to the extent that measurable photoactivity was only observed with DPC-C4-Ni. It showed the best-reported CO production rate of 2464± 40 mmol gNi-1 h-1 and selectivity greater than 95% in the flow conditions. The catalyst showed extraordinary stability (100 h).
Super-linear power law dependence on the light intensity (power law exponent of 5.6) with photocatalytic quantum efficiencies increased with an rise in light intensity and reaction temperature, while the kinetic isotope effect (KIE) in light (1.91) was higher than in the dark, confirmed the hot-electron mediated reaction mechanism. Ultrafast studies of hot-carrier dynamics proved the superfast electron injection from Au to Ni, populating the Ni reactor with charge carriers. Researchers observed a spectral signature of such an indirect charge generation due to hot electron transfer from the gold to the nickel. Finite-difference time-domain simulations also showed plasmon-induced high local field intensity enhancement in DPC-C4-Ni.
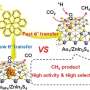
An in-situ DRIFTS study showed C=O stretching vibrations of linearly bonded CO atop Ni atom, while bridge carbonyl species formation was hindered. CO2 hydrogenation took place by direct dissociation path via linearly bonded nickel-CO. The linearly bonded CO on Ni sites of DPC-C4-Ni were weakly bonded due to its weak Ni-C bond. Hence CO desorption was efficient, restricting hydrogenation to methane, leading to more than 95% CO selectivity.
The high production rate and selectivity were due to Ni NPs being highly dispersed on black gold, providing a weakly bonded CO pathway, in addition to the excellent light-harvesting ability of black gold. Due to the excitation of electrons in the nickel d-band to higher energy level during plasmonic damping of black gold SPR, as well as to filling of Ni d-band due to hot electron transfer from black gold to Ni, Ni sites showed excellent activity even at smaller particle size.
The outstanding catalytic performance of black gold-Ni may provide a way to develop plasmonic catalysts for CO2 reduction and other catalytic processes using black gold.
More information: Rishi Verma et al, Nickel-Laden Dendritic Plasmonic Colloidosomes of Black Gold: Forced Plasmon Mediated Photocatalytic CO2 Hydrogenation, ACS Nano (2023). DOI: 10.1021/acsnano.2c10470
Journal information: ACS Nano
Provided by Tata Institute of Fundamental Research